Midwives are commonly asked by parents for advice on how they should clean their newborn infant and what should they use on his/her skin. The answers are not as straight forward as might be supposed. Inconsistent and conflicting professional opinion, overwhelming retail choice and advertising by product manufacturers all conspire to be a source of confusion for many families and midwives alike (Lavender et al, 2009). Skin care is a topic that this journal periodically revisits (Hale, 2008; Hughes, 2011; Jones, 2013), thus this article reviews some new evidence about the use of skin cleansing products.
Newborn skin
The human body is host to a complex population of symbiotic microorganisms that have a mutual, commensal and sometimes pathological relationship with their host. Collectively they are referred to as the human microbiome (Turnbaugh et al, 2007). The exact nature of the skin biome differs over time, body site and health status (Grice et al, 2009; Capone et al, 2011). However, little is known about how these microorganisms establish themselves during the first years of life (Grice and Segre, 2011; Callado et al, 2012). Nevertheless the roles of this microbiota in health and disease are becoming an increasingly important focus of research for its potential to have health promoting and therapeutic effects. It is widely understood that skin care choices and practises influence the integrity of skin barrier functionality (Garcia Bartels et al, 2010; Danby et al, 2013; Lavender et al, 2013). It is likely that everything which comes into contact with neonatal skin influences its maturation and the pattern of biome colonisation affecting health and pathology.
Newly born term infants have unique skin structure and physiology (Stamatas et al, 2010; Ludriksone et al, 2014) (Figure 1). While the skin is sufficiently mature to cope with the usual demands after birth it undergoes a period of rapid anatomical and physiological transformation (Stamatas et al, 2011; Visscher et al, 2011, Visscher and Narendran, 2014). Changes, particularly in relation to skin hydration, transepidermal water loss (TEWL) and the transition to a more acidic skin surface are in seen and used to examine the effects of different skin care routines and products on skin maturation and integrity. This process of maturation continues for several years (Paller et al, 2011; Stamatas et al, 2011) and this means that newborn skin is uniquely vulnerable to sensitisation, irritation and adverse alterations in skin barrier function.
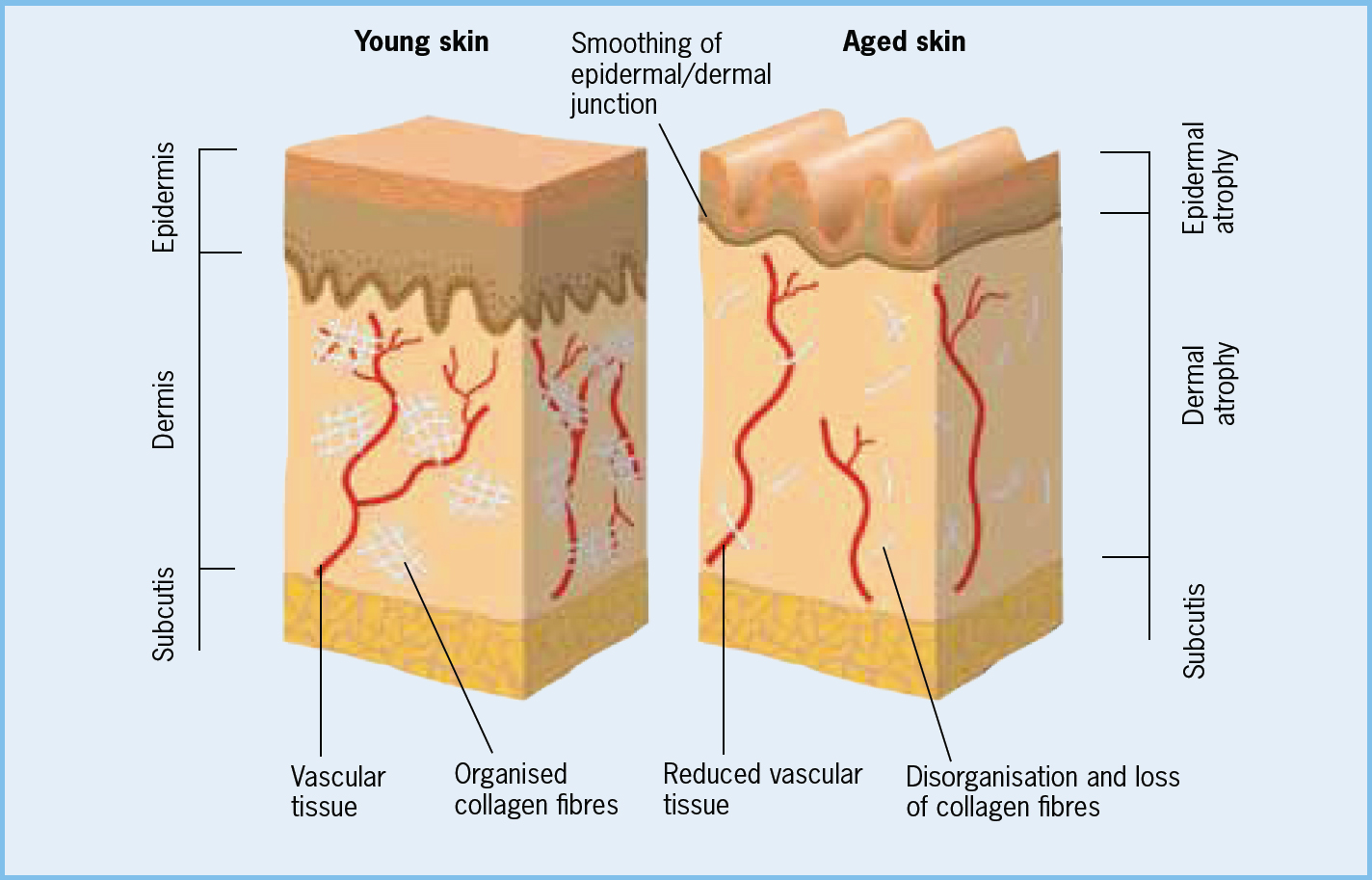 Figure 1. Physiology of the human skin
Figure 1. Physiology of the human skin
Current cleansing advice
Many maternity services' policies and individual midwives advocate a minimal or no product approach, using ‘plain water’ only, for skin cleansing, especially during the first weeks after birth. Proponents of water alone have sometimes firmly dismissed alternatives. This stance partly reflects a belief in the superiority of water to maintain skin barrier integrity, a rejection of the commercialisation of infant care and concern about the effects of pervasive marketing on parents. Concern about taking advantage of parents vulnerabilities are real and need wider debate, and undoubtedly avoiding unnecessary exposure to chemical reagents in skincare products and making parenthood less economically draining are attractive.
However, claims that using water alone is the gold standard of infant skin cleansing are not based on robust evidence. For example the National Institute for Health and Care Excellence postnatal care guidelines (NICE, 2006), now over 8 years old and under review, do not take into account recent research. The guidance in relation to neonatal skin hygiene was only graded as a good practice point; a recommendation based on opinion rather than other superior levels of evidence. The guideline recommended the avoidance of using medicated wipes, lotions and adding any cleansing agents to bath water yet confusingly also recommended that: ‘the only cleansing agent suggested, where it is needed, is a mild non-perfumed soap’ (NICE 2006: 31).
In the UK, responsibility for ensuring safe supplies of drinking water are set out in a complex regulatory framework. Despite this, the chemical makeup of water supplies delivered to the home can vary considerably. Two important water characteristics that can affect the skin are its acidity (pH) and hardness (a measure of the concentration of predominantly calcium and magnesium ions). These factors can have an effect on the cleaning abilities of water in general and some skin pathologies such as atopic dermatitis and nappy rash (napkin dermatitis) (McNally et al, 1998; Adam, 2008). Regulation sets the pH of water leaving treatment works between 6.5 and 9.5 but there are no regulatory limits set for water hardness. Locally, water pH can be affected by pipework corrosion, plumbing alterations, and household water softeners while the largest determinants of water hardness are geology and the primary source of the water.
Water does not effectively remove non-water soluble skin adherents. Using water alone to clean the skin can contribute to poor hygiene and in the nappy area leave deposits of faeces and urine in contact with the skin which might contribute to later skin health problems (Adam, 2008). Soaps are effective cleansers but are invariably highly alkaline, causing drying, irritation and stripping the skin of protective lipids. Products containing soap should be avoided in the newborn. One group consensus at a European meeting (Blume-Peytavi et al, 2009) stated that a pH neutral, mild liquid cleaner with a non-irritant (to eyes and skin) and hypoallergenic formulation was the preferred choice of infant cleansing product. Non-soap based cleaners containing synthetic detergents are widely used in manufactured personal care products. These detergents are effective cleansers, avoid some of the adverse effects of soaps and are known to be safe in adults. While these products seem to offer some advantages over water, a review carried out in 2010 failed to find robust evidence to support their use in the neonate (Crozier and Macdonald, 2010).
Despite the existence of influential guidelines like NICE (2006) and NHS Choices (2014) there is limited consensus on what are the most appropriate cleansing regimes and products to use in the neonatal period. This situation leaves midwives with unresolved challenges in defining optimal practice and how best to advise parents. Until recently, high quality evidence to guide practice in this area was not available. However, an emerging empirical research-base is now starting to provide answers for some of the many contentious questions around neonatal skin cleansing and care.
New research evidence
The objective of skin cleansing is to clean without removing protective surface lipids, affecting skin microbiota and changing pH. This is a considerable challenge as the composition and barrier integrity of skin is regionally variable and not static (Stamatas et al, 2011). Clearly a product intended for adults will not be appropriate for neonatal skin. Since their first inception decades ago, the formulation of infant skincare products has evolved as knowledge about the specific characteristics of infant skin and the needs of newborn skin cleansing has become available. Shops have a profusion of products all making claims and counter claims about what is best and least harmful. Sadly much of the research backing up these claims is either absent, weak or couched in language impenetrable to the non-specialist cosmetician or those unfamiliar with chemical nomenclature. This makes giving informed advice difficult and there is clearly a need for clinically useful information. The results of some studies including infants from birth in their sampling have begun to do this.
 Using water only is not effective at removing bodily wastes and this might explain why many mothers readily adopt wipes (Lavender et al, 2009)
Using water only is not effective at removing bodily wastes and this might explain why many mothers readily adopt wipes (Lavender et al, 2009)
One German study recruited 64 healthy term infants into a four-arm randomised trial comparing combinations of water, topical creams and two specific brand-named products between 1 and 8 weeks of age (Garcia Bartels et al, 2010). Infants exposed to the detergent gel cleanser had statistically lower pH over all sites compared to those who used water alone at 8 weeks of age, i.e. superior maturation. Other results reported comparable skin condition scores between all groups and similar patterns of change in skin function, suggesting equivalence between the four study arms. Another larger study (n=180) in the Philippines comparing water alone and two different named gel cleansers over 2 weeks drew similar conclusions about the safety and lack of adverse effects on skin integrity from using skin cleaning products (Dizon et al, 2010). Based on these two studies, skin cleansing regimes using specially formulated products did not adversely affect physiological skin maturation. However, these two studies had a number of methodological flaws, particular in relation to the lack of a predefined sample size and primary outcome; thus making any evidential claims unreliable (Crozier and Macdonald, 2010).
More recently in the UK the largest study to date recruiting over 300 healthy term newborns reported its results (Lavender et al, 2013). This study sought to evaluate the use of a branded wash product versus water alone and the effects these regimes had on skin barrier function. Based on pilot work (Lavender et al, 2011) a non-inferiority randomised clinical trial design was chosen. This research design, though methodologically complex and with a number of potential shortcomings, is becoming increasingly popular in health research (Head et al, 2012). It is particularly attractive in situations where using a placebo, like in a superiority clinical trial, is either not practical or ethically possible. Rather than seeking to determine which treatment delivers the best outcomes, like in a superiority trial, the aim in a non-inferiority study is to compare a standard treatment intervention, in this case water, with a comparator, the wash product to show that the comparator is no worse than the standard with regards to pre-specified criteria (Gupta, 2011). Importantly, the comparator will have similar efficacy but might offer other benefits over the standard such as, patient acceptability, fewer side effects and the like. In this sort of study it is crucially important for the study robustness to justify and predefine the primary outcome measure, sample size and the acceptable margin (non-inferiority margin) by which the comparison treatment is not unacceptably worse than the standard (Head et al, 2012). Unlike the Garcia Bartels et al (2010) and Dizon et al (2010) studies, Lavender et al (2013) describe and justify their design considerations in detail which is in accord with established reporting structures (Piaggio et al, 2012); this enriches the quality reporting of their findings.
The research (Lavender et al, 2013) found no differences in standard clinical measures and observations of skin condition (TEWL, pH, hydration, dryness and erythema) against their primary and secondary outcome measures. This led the authors to conclude that this wash product did not negatively affect skin barrier integrity and was therefore no worse (not inferior) than using water alone. Overall, it is difficult to generalise findings from these three studies but it seems likely that similar product formulations should be equally unproblematic. However, it is incumbent on practitioners to remain conversant with any changes in product design and interpret any claims with caution.
Nappy area cleansing deserves special consideration because of the problems and anxieties incorrect care can provoke. Using water only is not effective at removing bodily wastes and this might explain why many mothers readily adopt wipes (Lavender et al, 2009). Early formulations of wipe often contained alcohol and perfumes and were linked to adverse skin effects; this led to routine advice against their use in the neonate. More modern formulations often containing emollients have being shown in several randomised controlled trials from birth to be well accepted by parents, effective cleansers and equivalent or superior to water on skin integrity measures (Adam et al, 2009; Furber et al, 2012; Garcia Bartels et al, 2012; Lavender et al, 2012). These findings can offer reassurance to the many parents who chose to use such products from birth.
The desire by parents to protect and enhance their newborn's skin is understandable but how best to do this unclear. Appropriate cleansing and the maintenance of good hygiene are certainly contributory and some studies have shown that emollients are effective skin barrier enhancers (Telofski et al, 2012). However the most efficacious formulation of emollient remains elusive. Although natural oils like peanut are widely proscribed because of fears over sensitisation, some maternity services advocate that others, like olive oil, can be used on neonatal skin (Cooke et al, 2011). This is erroneous and potentially harmful advice. Olive oil contains high levels of oleic acid which can adversely affect skin barrier function and might be implicated in the development of skin conditions like atopic eczema (Danby et al, 2013). Conversely, evidence from studies of preterm and adult skin suggest that applying sunflower oil, which is low in oleic but high in linoleic acid, might be protective (Darmstadt et al, 2004; Danby et al, 2013). The evidence supporting this claim is limited and this has prompted attempts to systematically determine the effects of sunflower and other topical oils on infant skin function. A number of studies are ongoing and it is hoped that these results, when published, will help to develop conclusive guidelines on emollient use on healthy neonatal skin.
Implications for practice
The use of some specific branded formulations of skin cleansing products (washes and wipes) used from birth has being shown in large scale randomised trials to be no more harmful to skin barrier integrity and maturation than using water alone. This new evidence has begun to be incorporated into sources of professional guidance about newborn skin cleansing internationally. For example, in the USA the Association of Women's Health Obstetric and Neonatal Nursing (AWHONN 2013) and in the UK, the Royal College of Midwives (RCM) have supported this more evidence based approach (Lyon, 2014). It will be interesting to see how the influential NICE postnatal care guidelines currently under review (due December 2014) incorporate this new evidence. Regardless of how individual parents choose to clean their newborn's skin, it is incumbent on midwives to be conversant with current best evidence and provide impartial information and advice to enable parents to make truly informed choices.
Conclusion
Despite this new research the debate about optimal neonatal skin cleansing is unresolved and a number of clinically relevant questions remain, such as how best to ensure that parents make informed, as opposed to advertisement influenced, choices about skin cleansing and what is the best choice of emollient and how to use it. Nevertheless, in light of recent research health professional advice about the use of skin cleansing products needs to undergo a re-evaluation. There are now several examples of rigorously conducted research that provides a convincing body of evidence suggesting that specifically formulated skincare products when used properly can offer a safe and sometimes superior alternative, particularly in the context of effective cleansing and genital area hygiene. This information will assure parents that if they chose to use products, either from birth or later on, they are not causing harm.
Key points
- Midwives need to be familiar with the evolving anatomy and physiology of neonatal skin, skin pathology and have knowledge about current best evidence regarding skin cleansing in order to provide knowledgeable advice to parents about skin cleansing and oversee skin health
- Plain water is commonly advocated as the liquid of choice for cleaning newborns in the first weeks after birth, however this advice lacks a strong evidence base and recent research into skin cleansing products challenges this stance
- If parents wish to use a skin cleansing product from birth they can be confident that selecting one with a specific formulation like those that have undergone robust clinical study will not harm their newborn's skin

