Congenital heart disease (CHD) accounts for 3%–7.5% infant deaths in high-income countries (Knowles et al, 2014; Singh et al, 2014). For this reason, screening for CHD is included in newborn examinations. In the UK, screening for CHD forms part of the newborn and infant physical examination (NIPE), which screens for conditions of the eyes, heart, hips and testes. The NIPE is a two-stage screening examination that is performed within 72 hours of life and at six weeks of age (Public Health England, 2020). This paper will focus exclusively on the newborn physical examination (NPE).
Historically, in the UK, junior neonatologists performed this examination. However, it is increasingly becoming the midwife's role (Yearley et al, 2017). Recently, the Nursing and Midwifery Council ([NMC], 2019) released their new standards of proficiency for midwives. The skills and knowledge pertaining to the NPE will now be included in the pre-registration midwifery curriculum.
Auscultation of heart sounds is a key component of screening for CHD at the NPE to detect the presence of heart murmur. However, a newborn murmur does not always denote CHD but its presence triggers further investigation (Seignior, 2019). This is because newborn murmurs can be caused by the persistence of fetal cardiovascular structures, such as the ductus arteriosus (DA), during the first few days of life (Tucker Blackburn, 2018). Conversely, it has been found that the absence of heart murmur does not preclude critical CHD (Ainsworth et al, 1999; Wren et al, 2008). A newborn with duct-dependent critical CHD (CCHD) can be asymptomatic during the NPE and collapse days later. This is because while the DA is still open, the newborn circulation can bypass the cardiac malformation, masking the signs and symptoms of serious disease (Mellander, 2013; Singh et al, 2014).
Therefore, midwives who undertake the NPE must be aware of its limitations. Failure to detect the presence of CCHD at the NPE was the concern that triggered the author to conduct this literature review. The research question focused on the auscultation of heart murmur and its correlation with CHD. The following aims were identified:
- When screening for CHD in asymptomatic newborns, what is the significance of hearing a murmur?
- Can the NPE examiners identify the difference between innocent and pathological murmurs to identify correct care pathways?
- What is the value of pulse oximetry as an additional screening test?
- What is the relevance of the NPE timing relating to the sensitivity and specificity of the CHD screen?
- When heart murmurs are heard during the first day of life, what is the evidence for delaying discharge until after 24 hours?
Methodology and research design
A critical literature review was chosen because, when conducted with rigour, this can establish what is known or unclear on a topic (Grant and Booth, 2009; Aveyard, 2014). A narrative synthesis approach was chosen which is appropriate for diverse methodology and findings (Bettany-Saltikov, 2010).
Data and information sources
The following databases were accessed to source relevant articles: Medline, Cinahl, Science Direct, Intermid, Maternity and Infant Care, Cochrane Library, Science Direct and Scopus. Reference lists from retrieved articles were searched by hand.
Search strategy
Search components (Table 1) were based on the PICO framework (Guyatt et al, 2011). This framework focuses the question and identifies its parts for a literature search (Carman et al, 2013; Hewitt-Taylor, 2017). Search terms were combined with Boolean Operators and are shown in Table 2:
Table 1. Adapted PICO framework
| Population | Newborn babies |
| Intervention | Auscultation of heart murmur |
| Outcome | Congenital heart disease detection |
Table 2. Search terms used
| Search ID | Search terms |
|---|---|
| S1 Abstract (AB) | newborn OR neonat* OR infant* OR bab* |
| S2 (AB) | ‘heart murmur*’ OR ‘cardiac murmur*’ OR ‘heart sound*’ |
| S3 (AB) | ‘congenital heart disease’ OR ‘congenital heart defect*’ OR ‘congenital cardiac defect*’ OR ‘congenital cardiac disease’ OR ‘congenital heart malformation*’ OR ‘congenital cardiac malformation*’ |
Inclusion and exclusion criteria
All research papers on the topic that were written in English, from peer-reviewed academic journals between 1999–2019, were included. The wide time frame was chosen to include seminal work.
The following study types were retrieved:
- Systematic review
- Prospective (cohort) study
- Retrospective case or case-control study
- Cross-sectional study
- Survey
- Audit
A total of 56 papers were retrieved after removing duplicates (Figure 1). The retrieved papers were reviewed by reading abstracts. The author excluded 24 non-relevant papers. These included papers conducted in neonatal units, as participants would be preterm or unwell, and studies comparing the skills of neonatal doctors with paediatric cardiologists. The latter are not typically involved in newborn screening. In addition, there were 14 single case reports that lacked generalisability, two non-English studies and four from low-income countries. The latter were excluded as their health services are not comparable to ours.
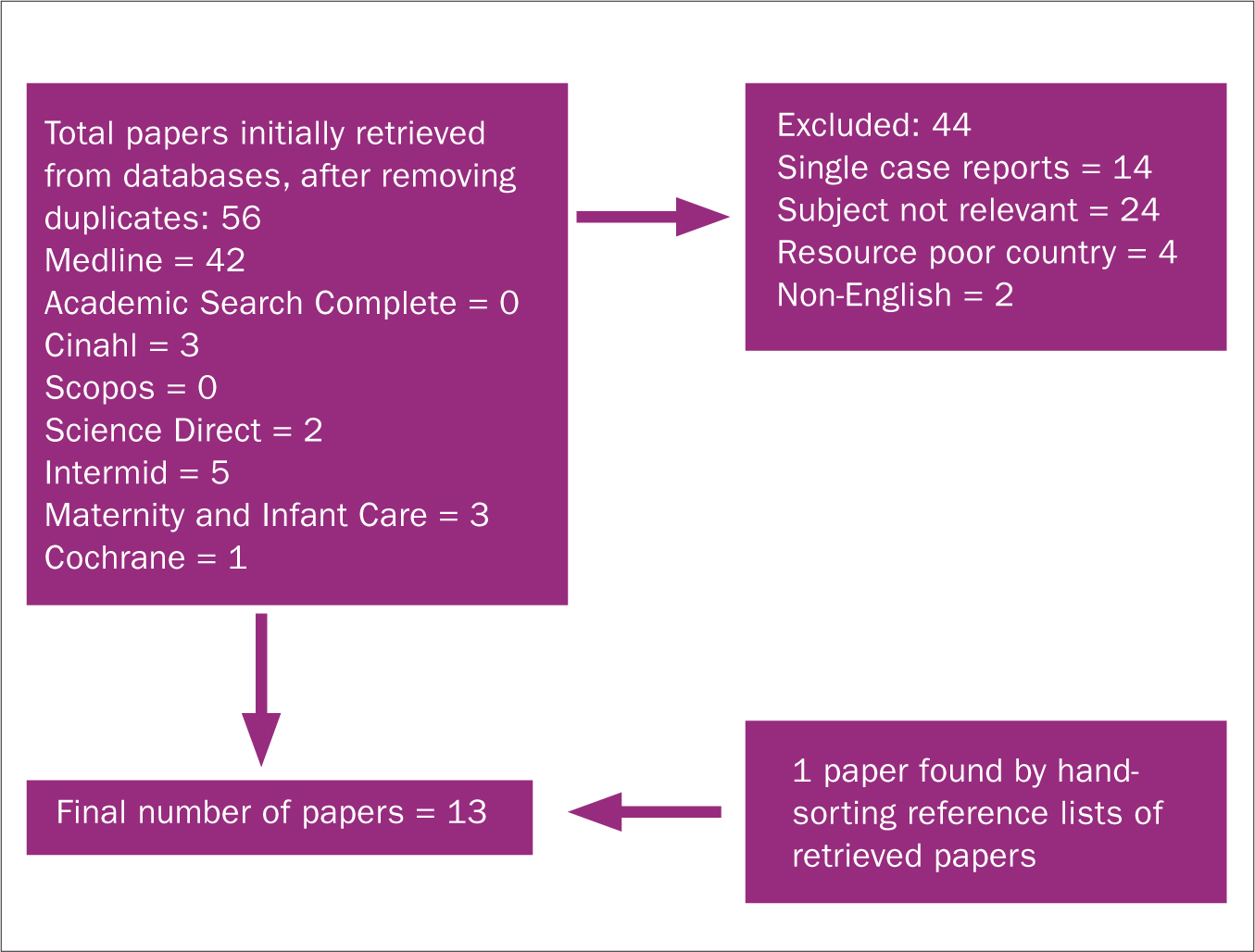 Figure 1. Flow chart summarising search strategy
Figure 1. Flow chart summarising search strategy
The remaining papers were obtained and scrutinised, including reference lists. One additional paper was found from this hand search. The author also found one Cochrane Review (Plana et al, 2018) about pulse oximetry screening for CCHD. Due to the immediate relevance and high quality of this review, no other literature on pulse oximetry was included.
Findings
The papers were analysed using McMasters' Quantitive Critical Appraisal Tool (Law et al, 1998). Tables were formulated, collating the main features of each study, to identify emergent trends (Tables 4–7). The author has sought to be consistent with terminology. Day 0 refers to the day of birth <24 hours, day 1 is 24–48 hours, day 2 is 48–72 hours.
Table 3. Correlation of heart murmur and existence of congenital heart disease (CHD): data from four retrospective studies
| Author | Prevalence of murmur % (n) terms | Prevalence of CHD as % (n) of newborns with murmur | Prevalence of CCHD as % (n) of newborns with murmur |
|---|---|---|---|
| Al-Ammouri et al (2016) | 1.65% (309) | 22% (68) | 2.6% (8) |
| Lardhi (2009) | 1.37% (87) | 42.5% (37) | 5.4% (4) |
| Rein et al (2000) | 0.8% (170) | 67% (114) | 3% (5) |
| Singh et al (2012) | 0.9% (205) | 40% (82) | 2% (4) |
Table 4. Significance of hearing a murmur as prediction of congenital heart disease (CHD): summary of main study features
| Study details | Key characteristics of study | Results/statistical analysis | Key study findings and recommendations | Limitations of study |
|---|---|---|---|---|
| Ainsworth et al (1999) Country: UK Prospective study over two years N=7 204 newborns | Aim: identify prevalence and significance of murmurs detected on the newborn physical examination (NPE) Examiner level: paediatric senior house officers (SHOs) with identical training Location: postnatal ward NPE timing: day 0 or day 1 postnatal Cohort demographics: >35 weeks, asymptomatic newborns on postnatal ward Reference test: echocardiogram | Prevalence of murmur: 0.6% (n=46) 54% (n=25) of newborns with murmur had CHD 9% (n=4) had critical CHD (CCHD) 60% (n=15) of newborns with CHD had ventricular septal defect (VSD) Significant or critical CHD missed at NPE due to no murmur: 0.4% (n=32) Sensitivity of the NPE for detection CHD: 44% (95% CI 31%−58%); specificity: 99.7% Presence of murmur has positive predictive value of 54% for CHD (95% CI 39%−69%) | Prevalence of murmurs detected at newborn examination is <1% Approximately 50% are due to CHD. The presence of heart murmur should trigger early echocardiography. Absence of murmur does not exclude CCHD | Examinations conducted on noisy postnatal ward; potentially affecting detection of soft murmurs. This had potential to skew prevalence figure for CHD upwards, as a proportion of newborns with murmurs |
| Al-Ammouri et al (2016) Country: Jordan Retrospective study over seven years N=309 newborns with heart murmur | Aim: whether murmurs detected at the NPE need follow-up echocardiography Examiner level: paediatric resident Location: postnatal ward Echocardiogram timing: day 0 or day 1 postnatal Cohort demographics: asymptomatic newborns with murmur, gestation not stated. However, all were from postnatal ward; likely late preterm/term Reference test: echocardiogram | Prevalence of murmur: 1.65% (n=309) 22% (n=68) of newborns with murmur had CHD 2.6% (n=8) of newborns with murmur had CCHD 53% (n=36) of newborns with CHD had VSD 75% (n=27) of VSDs were small muscular defects PDA excluded from statistics for CHD | The presence of heart murmur should trigger echocardiography before discharge from hospital | Gestation of newborns in study not explicitly given |
| Lardhi (2010) Country: Saudi Arabia Retrospective study over four years N=6 333 newborns | Aim: determine the prevalence and importance of murmurs detected on NPE Examiner level: paediatric residents Location: postnatal ward or well-newborn nursery NPE timing: day 0 Cohort demographics: term, asymptomatic newborns in well-baby nursery or postnatal ward Reference test: echocardiogram | Prevalence of murmur: 1.37% (n=87) 42.5% (n=37) of newborns with heart murmur had CHD 62% (n=23) of newborns with CHD had VSD ‘Most’ VSDs were small muscular defects 5% (n=4) of newborns with murmur had CCHD | 42.5% newborns with murmurs have CHD. The presence of heart murmur should trigger early echocardiography | Three newborns with PDA were included, but this is not abnormal within first 24 hours |
| Meberg et al (1999) Country: Norway Prospective study over 15 years N=35 218 newborns | Aim: identify how much CHD missed by NPE; are two examinations better than one? Examiner level: consultant neonatologists, SHOs and registrars Location: well-baby nursery NPE timing: first half of study period = day 1 and day 5; second half of study period = day 2 Cohort demographics: gestation not stated. However, since all newborns from well-baby nursery, likely late preterm/term Reference test: echocardiogram | Overall prevalence of CHD: 1% CHD missed by NPE: 24% (n=84) 48% (n=40) of missed cases were significant VSD 8% (n=7) of missed cases presented unwell requiring urgent cardiac surgery Detection rate did not change when one examination was performed compared to two (p<0.05) | Early detection is poor for left obstructive CHD eg aortic stenosis (AS), coarctation of aorta (COA) and large VSD. One examination is as safe as two but should be undertaken as near to discharge as possible. The presence of heart murmur should trigger early echocardiography | Gestation of newborns in cohort not explicitly given. Level of examiners was mixed ie consultant/registrar/SHO. Environmental factors (noisy ward) stated as possible reason for failure to detect CHD that usually presents with murmur |
| Mirzarahimi, et al (2011) Country: Iran Cross-sectional study over one year N=2 928 newborns | Aim: determine prevalence of heart murmur in newborn and its correlation with CHD Examiner level: paediatrician – does not state which level Location: hospital nursery NPE timing: day 0 Cohort demographics: all newborns born in hospital who were well enough to be in the nursery: 59.6% >35 weeks, 40.4% <34 weeks including 19.1% <29 weeks Reference test: echocardiogram | Prevalence of murmur: 3.1% (n=91) 52% (n=47) of newborns with heart murmur had CHD 34% (n=16) of newborns with CHD had VSD No significant relationship found between birth weight and CHD (p=0.4) No significant relation found between gestation and CHD (p=0.8) | The presence of heart murmur should trigger early echocardiography | 40% (n=19) newborns in the study were less than 34 weeks, including some extremely preterm (29 weeks). This could introduce bias as preterm babies often have persistence of fetal cardiovascular structures. The researchers included PDA in their CHD statistics. This constituted 11% (n=10) of forms of CHD recorded |
| Patton and Hey (2006) Country: UK Prospective audit over eight years N=14 572 newborns | Aim: assess what proportion of CHD can be detected at the NPE when examination is performed by small, stable clinician group Examiner level: nurse practitioner Location: postnatal ward NPE timing: day 1 Cohort demographics: asymptomatic newborns >36 weeks' gestation Reference test: echocardiogram. All newborns followed up by accessing congenital anomaly register, mortality register | 1.2% (n=176) newborns born over audit period had CHD Last three years of study: Prevalence of murmur found in asymptomatic newborns was 10.7% (n=515) on day 1 after birth 4.2% (n=203) still had a murmur at review on day 7 13% (n=67) of newborns with murmur had CHD confirmed by echocardiogram 11% (n=20) of newborns with CHD had clinically significant forms. Four newborns with CCHD were missed at the NPE in first 3 years of the study. None were missed subsequently Accuracy of NPE assessment for CHD for last three years of study: sensitivity: 94.3% (CI 89.5%−99.2%); specificity: 97.7% (CI 97.3%−98.0%) | Whether examiner has medical or nursing background is not as important as experience and skill of examiner in auscultation. Parent education is essential, given that some newborns with CCHD do not present with heart murmur at time of the NPE | |
| Rein et al (2000) Country: Israel Retrospective study over 3 years N=170 newborns referred for echocardiogram | Aim: to find cause of asymptomatic heart murmur by reviewing pre-discharge echocardiography NPE examiner level: ‘attending neonatologist’ likely to be junior. Location: postnatal ward NPE timing: day 1 Echocardiogram timing: between 1−5 days Cohort demographics: asymptomatic term newborns with heart murmur | Echocardiography findings for 170 newborns referred with asymptomatic murmur: 13% (n=22) had normal heart 67% (n=114) had CHD 3%(n=5) had CCHD 37%(n=54) of the CHD cases were VSD, including small insignificant lesions | There is a significant chance that asymptomatic newborns with heart murmurs have CHD. The presence of heart murmur should trigger early echocardiography | High prevalence of CHD as proportion of newborns with murmurs may relate to junior status of examiner whereby softer murmurs were missed. Statistic for CHD presented in abstract was 86%. This included 23% (n=34) newborns with PDA |
| Singh et al (2012) Country: UK Retrospective study over three years N=205 newborns referred for echocardiogram Who had asymptomatic murmur | Aim: review findings of pre-discharge echocardiography for newborn heart murmurs Examiner level: neonatologist trained in echocardiography Location: postnatal ward NPE timing: days 0−1 Echocardiogram timing: >48 hours Cohort demographics: asymptomatic newborns with murmur. Gestation likely late preterm/term | Echocardiography findings for 205 newborns with asymptomatic murmur: 26% (n=53) had normal heart 38% (n=78) had minor structural cardiac defects 34% (n=70) had transient circulatory changes such as PDA 2% (n=4) had CCHD. | The presence of heart murmur should trigger echocardiography before discharge from hospital. This allows prompt diagnosis so that parents' anxiety is alleviated. It also provides triage for newborns who require urgent treatment | Gestation of newborns not explicitly given |
Table 5. Accuracy of auscultation relating to skill/experience of examiner: summary of main study features
| Study details | Key characteristics of study | Results/statistical analysis | Key study findings and recommendations | Limitations of study |
|---|---|---|---|---|
| Ageliki et al (2011) Country: Greece Prospective study over 1 year N=169 newborns with heart murmurs | Aim: compare auscultation abilities of paediatric trainees and senior neonatologists Examiner level: paediatric trainees and senior neonatologists. They were asked to identify whether murmurs were innocent, possibly pathological or pathological Location: postnatal ward (n=118), NICU (n=25) and OPD (n=26). NPE timing: newborns >24 hours (1−28 days; median age 5 days) Cohort demographics: asymptomatic newborns 32−41 weeks' gestation Reference test: echocardiogram | 32.6% (n=55) of newborns with murmur had CHD 55% (n=30) of newborns with CHD had VSD. 4.1% (n=7) had CCHD Paediatric trainees' ability to identify pathological/possibly pathological murmur: sensitivity 94.6% (CI 83.9–98.6); specificity: 64.0% (CI 54.5–72.7) Senior neonatologists' ability to identify pathological/possibly pathological murmur: sensitivity 98.2% (CI 89.0–99.9); specificity 71.1% (CI 61.7–79.0) Paediatric trainees were less able to distinguish pathologic heart murmur (p=0.007) | Paediatric trainees need to have access to a second opinion from a fully trained relevant physician. Level of experience is important for differentiating between innocent and pathological murmurs. Overall, the diagnostic value of cardiac auscultation is limited and depends on the clinician's experience | Wide confidence intervals for some statistics |
| Farrer and Rennie (2003) Country: UK Prospective study over 2 years N=112 newborns with heart murmurs | Aim: to assess paediatric SHOs' ability to discern whether a murmur is innocent or pathological Examiner level: SHOs asked to decide on appropriate care pathway depending on quality of murmur Location: postnatal ward NPE timing: >24 hours. Mean age 1.5 days SHOs allocated newborns to one of four groups: G1: immediate admission NNU (cyanosis or signs cardiac failure) G2: family history or worrying antenatal scan; refer to senior neonatologist G3: murmur deemed pathological; refer to next cardiac clinic G4: murmur deemed innocent; non-urgent referral to outpatient department (OPD) Cohort demographics: asymptomatic newborns 35−42 weeks Reference test: echocardiogram | Prevalence of murmur = 1.38% (n=112) 23% (n=21) of newborns with murmur had CHD. However, 48% (n=10) had minor conditions resolving by 6 months G1: 12 newborns; 11 had major or critical CHD G2: 8 newborns: all had normal heart G3: 9 newborns: 4 had CHD, 5 had normal heart G4: 91 newborns: 69 seen OPD: 63 had normal hearts; 6 had small VSD/PDA/mild pulmonary stenosis. 22 newborns did not attend but no adverse sequelae reported by GP Accuracy of the SHOs examination for detecting CHD: sensitivity 71%; specificity 91% Positive predictive value: 71%; negative predictive value: 91% | SHOs can assess the clinical significance of neonatal murmurs and decide on appropriate follow-up | SHOs had a varying amount of experience. Some had 6 months, others had postgraduate paediatric qualifications and long experience |
| Khalilian et al (2016) Country: Iran Cross sectional study over 1 year N=137 newborns with heart murmurs | Aim: to assess examiners' ability to differentiate between innocent and pathological murmurs Examiner level: neonatologist, level not stated Neonatologists auscultated and categorised murmurs as ‘probably innocent’ or ‘probably pathological’ Location: most from well-baby nursery, some from NICU Examinations performed in quiet room NPE timing: Within 48 hours of birth Cohort demographics: asymptomatic newborns 35−42 weeks Reference test: echocardiogram | Prevalence of murmur = 1.92% (n=137). 49% (n=59) of newborns with murmur had CHD Accuracy of neonatologist to determine if murmur pathological: sensitivity 79.7%; specificity 88.5% Positive predictive value 87%; negative predictive value 81.8% FPR 11.5%; FNR 20.3% Significant relationship found between soft murmurs (grades l/ll) and a normal heart (p<0.001) 41/42 newborns whose murmurs were described as pansystolic had CHD. There was a significant relationship between murmur timing and existence of CHD (p<0.001) | Based on the high FPR and FNR of clinical assessment, the presence of heart murmur should trigger early echocardiography | Level of examiner not given |
Table 6. Value of pulse oximetry in screening for congenital heart disease (CHD): summary of main study features
| Study details | Key characteristics of study | Results/statistical analysis | Key study findings and recommendations | Limitations of study |
|---|---|---|---|---|
| Plana et al (2018) Countries: UK (n=5), Italy (n=2), US (n=3), Australia (n=1), China (n=1), Germany (n=1), Mexico (n=1), Norway (n=1), Poland (n=1), Saudi Arabia (n=1), South Africa (n=1), Sweden (n=1), Switzerland (n=1) and Turkey (n=1). Systematic review: n=21 studies, 457 202 newborns | Aim: systematic review assessing the diagnostic accuracy of pulse oximetry screening for critical CHD (CCHD) Cohort: term and late preterm asymptomatic newborns born in hospital Reference standards: echocardiogram and clinical follow up for 28 days, post-mortem findings, congenital anomaly databases | Pulse oximetry is a highly specific screen with moderate sensitivity and a low FPR Sensitivity for detection of CCHD: 76% (95% CI 69.5−82.0) Specificity for detection of CCHD: 99.9% (95% CI 99.7−99.9) FPR: 0.14% ((95% CI 0.07−0.22) No significant difference whether the screen was performed before or after 24 hours, except FPR lower when the screen was performed >24hrs: >24hrs: FPR 0.06% (95% CI 0.03−0.13) <24hrs: FPR 0.42% (95% CI 0.20−0.89) (P=0.027). | Current evidence supports the use of pulse oximetry as a routine screening test for CCHD in asymptomatic newborns before discharge home. The lower FPR when the screen is performed >24hrs needs to be balanced with the possibility of newborns becoming symptomatic before screening can take place | Definition of CCHD in the literature was highly variable. The low number of CCHD cases within the studies means that sensitivity precision is low. Incomplete follow up of newborns who screened negative in some studies also affected this statistic |
Table 7. Management of newborns with murmurs: Summary of main study features
| Study details | Key characteristics of study | Results/statistical analysis | Key study findings and recommendations | Limitations of study |
|---|---|---|---|---|
| Shenvi et al (2013) Country: UK Telephone survey and systematic review of management of asymptomatic newborns with heart murmurs N=193 neonatal units. | Aim: to review current evidence and practice in the management of asymptomatic newborns with heart murmurs. Participation from 132 (68%) of neonatal units in the UK. Survey conducted with either neonatal speciality registrar (SpR), ANNP or consultant The methodology for the systematic review was described in detail | Management by neonatal units of asymptomatic newborns with murmurs: 94% (n=124) performed pulse oximetry 76% (n=100) measured 4-limb BP 27% (n=36) performed a CXR 39% (n=52) performed an ECG 65% (n=86) units reported that in-house echocardiography services are provided Only 16.6% (n=22) performed echocardiography on newborns with murmurs before discharge | No evidence was found in the systematic review to support the routine use of 4-limb BP, CXR and ECG in the investigations for CHD. Clinical examination and use of pulse oximetry are crucial for identifying CHD in an asymptomatic term newborn with murmur before discharge | No mention of the NPE timing or discharge timing relating to management of asymptomatic babies with murmur |
When screening for CHD in asymptomatic newborns, what is the significance of hearing a heart murmur?
A total of eight papers addressed this as the main outcome; three were prospective studies, four retrospective and one cross-sectional (Table 4). All studies used echocardiography as a reference test following murmur identification. The large, prospective study by Ainsworth et al (1999) is regarded as seminal. In their two-year study, researchers collected outcome data for 7 204 asymptomatic newborns receiving the NPE via their records and the regional paediatric cardiology database. All examinations were conducted by senior house officers (SHOs) who received the same training, in the postnatal ward, within 48 hours of birth, indicating strong internal validity. Ainsworth et al (1999) found that 0.6% (n=46) of the babies had murmurs. Of this number, 54% (n=25) had CHD; 9% (n=4) had CCHD. Murmur prevalence was low compared to other studies (Farrer and Rennie, 2003; Patton and Hey, 2006; Al-Ammouri et al, 2016). A stated limitation of the study was that examinations were conducted on a busy ward, where auscultation of softer murmurs may be difficult. Ainsworth et al (1999) reported that 0.4% (n=32) of newborns in the study had a normal NPE but were subsequently diagnosed with significant or critical CHD before they were one year old. Thus, the absence of a murmur does not preclude the possibility of CCHD; an important finding for all clinicians involved in the NPE.
Meberg et al (1999) prospectively registered 35 218 newborns over 15 years. Examiners were consultants, registrars and SHOs. Examinations were conducted in the well-baby nursery. The focus was the number of cases of CHD missed and whether two examinations were better than one. During the first seven years of the study, newborns had examinations on days 1 and 5; during the second seven years, one examination was performed on day 2. CHD prevalence of 1% (n=353) was found. Of this number, 76% (n=268) were detected by examination, while 24% (n=84) were missed and diagnosed later in infancy. Cases missed equated to 0.3% of all newborns in the study. Some 48% (n=40) of those missed had a significant ventricular septal defect (VSD) and 8% (n=7) presented unwell at hospital, requiring urgent cardiac surgery. There was no statistical difference in the number of missed CHD cases, whether one or two examinations were conducted (p<0.05). Meberg et al (1999) commented that some newborn heart murmurs could have been missed due to ward noise. Compared to Ainsworth et al (1999), there were fewer missed cases, perhaps because senior neonatologists performed most examinations.
Mirzarahmi et al (2011) conducted a cross-sectional study over one year with 2 928 newborns. Levels of paediatrician performing examinations were not stated. Examinations took place on day 0. Murmurs were reported in 3.1% (n=91) of newborns, of whom 51.6% (n=47) had CHD. However, several factors affected validity. Firstly, 40% (n=19) of newborns included were <34 weeks. Heart murmurs are common in very preterm babies due to persistence of fetal physiology (Hoffman and Kaplan, 2002). The researchers included patent ductus arteriosus (PDA) within the figures for CHD. This can be a physiological variant for newborns, particularly for those born preterm. Hence, this CHD percentage may be inflated.
Patton and Hey (2006) conducted a large prospective audit over eight years, focusing on nurse practitioners' ability to identify CHD in asymptomatic newborns through auscultation at the NPE. This was the only study found that focused on non-medical practitioners. The audit tracked the detection rate of a small, stable team. The researchers tracked all newborns examined via the regional paediatric cardiology database. Nurses' detection skills developed over time. Over the last four years of the study, sensitivity of the NPE for detection of CHD was 94.3% (CI 89.5%–99.2%); specificity 97.7% (CI 97.3%–98.0%). Four newborns with CCHD were missed at the NPE during the first three years of the study but none subsequently. Interestingly, murmur prevalence in the last three years of the study was 10.7% (n=515). This was higher than in the other papers reviewed. This may be because the nurse practitioners became highly skilled in auscultation over the eight years, hearing the softest murmurs. Thus, in terms of auscultation, experience may be more important than professional background. Four retrospective studies addressed the significance of heart murmur in asymptomatic newborns as their main outcome.
Across the four retrospective studies, considerable heterogeneity was noted for the correlation between newborn heart murmur and CHD (Table 3). The range for this correlation from all studies included in this review was 13%–67%. However, this range reflects differences in CHD categorisation. Some authors included conditions relating to transitional physiology in newborns, such as PDA, thus inflating CHD statistics (Table 4). In addition, VSD was found to be categorised in varying ways. This condition comprised 48%–62% of defects found. VSD ranges from large defects, which cause long-term morbidity, to clinically insignificant, small ‘muscular’ VSD, which close spontaneously within the first few months of life (Ainsworth et al, 1999; Patton and Hey, 2006; Wren et al, 2008). The inclusion of clinically insignificant VSD potentially inflates the prevalence figure for CHD.
An important statistic for NPE practitioners relates to CCHD. Early detection of newborns with CCHD is a main aim of newborn screening, enabling urgent treatment (Knowles et al, 2014). CCHD prevalence in newborns with heart murmurs was found to be between 2%–9% in the papers included in this review.
What has not been included in the studies reviewed so far is the relevance of murmur grade for detecting CHD. How murmurs are graded according to sound and timing is explained by Frommelt et al (2004). The author was interested in reviewing papers about examiners' ability to differentiate between pathological murmurs (related to CHD) and innocent murmurs (related to normal newborn physiology), according to their sound and timing.
Can NPE examiners identify the difference between innocent and pathological murmurs to identify correct care pathways?
Three papers addressed this aim. Farrer and Rennie (2003) conducted a prospective study on SHOs' ability to assess the quality of neonatal heart murmurs. The SHOs had varied levels of experience. Following the NPE, newborns were assigned to one of four care pathways (Table 5). Examinations were conducted after 24 hours and newborns had to be asymptomatic. The researchers analysed the data in relation to care pathway assigned and diagnosis revealed by echocardiogram. SHOs appropriately referred all newborns with murmurs who had CCHD for immediate assessment; this was due to other worrying physical signs at the NPE. Sensitivity for discerning the clinical significance of murmurs was 71%; specificity was 91%. The researchers felt that these outcomes validated that SHOs can assess neonatal murmur adequately. However, there was no explanation of how this judgement was made. The sample was small, and possibly underpowered to suggest generalisability.
Khalilian et al (2016) conducted a cross-sectional study over one year to identify whether two neonatologists could discriminate between innocent and pathological murmurs (Table 5). Sensitivity for identifying pathological murmurs was 79.7%; specificity was 88.5%. The positive predictive value was 87%; negative predictive value was 81.8%. Again, the sample was small, affecting generalisability. These statistics are comparable to those from Farrer et al (2003). However, the researchers' conclusions were different. Khalilian et al (2016) recommended echocardiogram for all newborns with murmurs, due to the high false-positive rate (FPR) and high false-negative rate (FNR) from clinical assessment alone.
Khalilian et al (2016) found a significant association between soft, early systolic murmurs of grades l or ll and normal hearts. In all cases, (n=61) where echocardiogram showed normal hearts, murmurs were graded l or ll (p<0.001). Likewise, 41 out of 42 newborns whose murmur was described as pansystolic had CHD.
Ageliki et al (2011) compared the abilities of paediatric trainees with senior neonatologists in discerning whether murmurs found at the NPE were innocent, possibly pathological or pathological, as confirmed by echocardiogram (Table 5). Paediatric trainees' sensitivity was 94.6% (CI 83.9%–98.6%); specificity 64.0% (CI 54.5%–72.7%). Senior neonatologists' sensitivity was 98.2% (CI 89.0%–99.9%); specificity 71.1% (CI 61.7%–79.0%). The researchers concluded that junior clinicians must refer to senior colleagues to confirm clinical findings.
What is the value of pulse oximetry as an additional screening test for CHD?
Plana et al (2018) investigated whether universal pulse oximetry screening would increase CCHD detection in asymptomatic newborns (Table 6). Studies that performed the screen before 24 hours were compared with those performed afterwards. Use of pre- and post-ductal pulse oximetry was compared to testing with one post-ductal reading. Overall, sensitivity of 76% (CI 69.5%–82.0%) and specificity of 99.9% (CI 99.7%–99.9%) were found. The FPR before 24 hours was 0.42% and after 24 hours was 0.06%.
The researchers recommended universal pulse oximetry screening based on the high specificity of the test and moderate sensitivity. There were wide confidence intervals for the sensitivity value. This may reflect the scarcity of the condition within the denominator population and the varying methods used to follow up newborns after the pulse oximetry test.
No significant difference was found in sensitivity and specificity for CCHD detection by pulse oximetry relating to newborn age. However, there was a higher FPR when the test was performed under 24 hours which may have implications for increased parental anxiety, delayed discharge and additional investigations (Table 6). No statistical difference in sensitivity or specificity was found whether two readings (pre- and post-ductal) or one post-ductal reading was taken. This may have important implications for clinicians regarding the time needed to perform the screen.
What is the relevance of the NPE timing relating to the sensitivity and specificity of the CHD screen? When heart murmurs are heard during the first day of life, what is the evidence for delaying discharge until after 24 hours?
The relevance of the NPE timing was not a main outcome in any of the studies retrieved. Searches that combined search terms related to the NPE timing with CHD found no results.
The NPE timing in the included studies varied from <24 hours (Lardhi, 2010; Mirzarahimi et al, 2011) to a median age of five days post-birth (Ageliki et al, 2011). The only survey included in this review was a telephone survey of all 193 neonatal units in the UK, combined with a systematic review of the management of asymptomatic newborns with heart murmurs (Shenvi et al, 2013; Table 7). Remarkable variation in management was found between the 68% (n=132) of units that responded. Notably, only 16.6% (n=22) performed echocardiogram on newborns with murmurs before hospital discharge. Unfortunately, the NPE timing was not recorded. Two main reasons for delaying discharge until after 24 hours were found in background literature reviewed by the author:
- This reduces pressure on resources because a proportion of murmurs related to transitional physiology will have disappeared (Gladman, 2012)
- It allows time for signs and symptoms of duct-dependant CCHD to manifest, as the DA closes (Green and Oddie, 2008).
It is known that closure of the DA may not occur until 2–7 days (Arlettaz et al, 1998; Hoffman and Kaplan, 2002). Therefore, delaying discharge to after 24 hours is insufficient for identifying all newborns with duct-dependant CCHD. The author has not been able to uncover the origin of this time given to parents in the literature reviewed. However, it is fortunate that, in the UK, community midwives visit the day after mothers and newborns return home (Royal College of Midwives, 2014). Thus, all newborns are examined at this time, providing further opportunity to monitor for signs of CCHD.
Discussion
This critical review explores the significance of hearing a heart murmur at the NPE relating to CHD detection in asymptomatic newborns. The research reviewed was of varied methodology and quality, and is relatively sparse, given that it spans the past 20 years.
The term CHD embraces a range of conditions, some of which are clinically insignificant. The importance of identifying newborns with CCHD has been highlighted, as this is life-threatening. CCHD includes most duct-dependent conditions, meaning symptoms do not manifest until the DA closes, often after hospital discharge (Knowles et al, 2005). Also of concern are significant forms of CHD that cause long-term morbidity if undetected (Knowles et al, 2014).
Heart murmur prevalence at the NPE was found to range from 0.6% (Ainsworth et al, 1999) to 10.7% (Patton and Hey, 2006). If the statistic from Patton and Hey is excluded, this range is 0.6%–1.68% (Al-Ammouri et al, 2016). Prevalence was lower than has been described elsewhere (Frommelt et al, 2004; Gladman, 2012). This could be explained by noisy ward environments and junior examiners in some of the studies included, whereby soft murmurs were not detected. The small group of examiners in the Patton and Hey study became highly experienced, perhaps explaining the higher prevalence of murmurs found. Their findings might not be generalisable, as in most maternity units, the NPE is performed by SHOs or midwives with varied experience (Rogers et al, 2015).
Considerable heterogeneity (13%–67%) was also found in CHD prevalence as a percentage of newborns with murmurs. The higher statistics may include small muscular VSDs that are clinically insignificant plus conditions related to transitional physiology in newborns, such as PDA. CCHD as a percentage of newborns with murmurs ranged from 2%–9%; far higher than anticipated. The 9% was found by Ainsworth et al (1999). However, in that study, all examinations were on a noisy postnatal ward, meaning soft murmurs might have been missed, potentially skewing this statistic upwards.
The author analysed three studies about examiners' skill in identifying murmur grade and timing, and its relation to transitional physiology in newborns (innocent) or to CHD (pathological). Sensitivity of auscultation for CHD identification ranged from 71%–98%; specificity 64%–91%. However, there were wide confidence intervals due to the small numbers involved and the scarcity of the conditions being screened for in the denominator population. Ageliki et al (2011) found that specificity for junior paediatricians' ability to identify pathological murmurs was lower than for senior neonatologists, suggesting that detection of pathological murmurs improves with experience. Farrer and Rennie (2003) concluded that SHOs had sufficient auscultation skills to determine newborn care pathways, although this seemed to be based on the researchers' judgement alone.
Khalilian et al (2016) did not make the examiners' level explicit. They concluded that clinical judgements had high FPR and FNR levels, and that echocardiography was necessary. Interestingly, 100% (n=61) of newborns with grade l or ll murmurs had normal echocardiograms (p<0.001). This aligns with Arlettaz et al (1998), who found a strong correlation between murmur grading and CHD presence or absence.
Overall, this evidence suggests if a murmur is identified at the NPE, newborns should be reviewed by a senior colleague before a decision is made about urgency of echocardiogram. To contextualise the significance of newborn heart murmurs, one should consider the missed CHD cases at the NPE where no murmurs were found. Three studies tracked outcomes for all newborns examined (Ainsworth et al, 1999; Meberg et al, 1999; Patten and Hey, 2006). Ainsworth et al found that in addition to the 0.6% (n=46) newborns with CHD who had murmurs, a further 0.4% of cohort (n=32) had no murmur recorded at the NPE but were diagnosed with significant or critical CHD later in infancy. The researchers found that the NPE detects only 44% of CHD which presents in the first year of life.
Meberg et al (1999) found that 24% (n=84) of newborns diagnosed with CHD within the first year had no murmur found at the NPE and were missed at that time. Some 8% (n=7) presented in a collapsed state at hospital requiring cardiac surgery.
Patten and Hey (2006) had fewer missed cases at the NPE found during their eight year audit; missing less than 10% (n=26). This included four cases of CCHD that were missed within the first three years. Their higher detection rate was attributed to the small yet stable team of experienced examiners and their robust referral system.
An interesting comparison between these studies is the NPE timing. Examiners in the study by Meberg et al (1999) and Patten and Hey (2006) missed a smaller percentage of newborns with CHD after conducting the NPE on days 1 or 2. Ainsworth et al (1999) performed the NPE on either day 0 or day 1, supporting the theory that a NPE conducted after 24–48 hours may be less likely to miss CHD.
This review has found that presence of heart murmur raises the likelihood of CHD in newborns from a background prevalence of 0.6%–0.9% to between 13%–67% (Rein et al, 2000; Patton and Hey, 2006). However, as discussed, the higher statistics include clinically insignificant cases. The likelihood of newborns with murmurs having CCHD rises from a background of 0.1% (Richmond and Wren, 2001) to 2%–9% (Ainsworth et al, 1999; Singh et al, 2012). However, if the softer murmurs were not heard by the examiners in these studies, due to busy ward environments, then the percentage for CCHD found may be inflated.
The findings of the Cochrane Review (Plana et al, 2018) were significant in terms of the value of pulse oximetry, which increases the sensitivity of the screen for CCHD at the NPE. Most forms of CCHD are associated with hypoxaemia which can be detected through pulse oximetry. It has been found that clinicians are poor at detecting cyanosis in newborns through visual inspection alone (O' Donnell et al, 2007).
No definitive answer about the optimum timing of the NPE for detecting CHD was found. From evidence reviewed here, it may be preferable after 24–48 hours. This will maximise detection of CCHD cases while still in hospital and reduce the FPR related to transitional physiology after birth. However, such extended postnatal stays are rare now in the UK, if mother and newborn appear well. It is likely the NPE timing will continue to be governed by hospital discharge which currently can be as soon as 2–6 hours after birth (RCM, 2014). While some maternity units arrange for newborns to receive their NPE the following day in the community, most do not have enough NPE-trained midwives to do this (Rogers et al, 2015).
Regarding newborns who have a murmur found at the NPE on day 0, it is not possible to state definitively that a 24-hour stay in hospital is evidence-based. While this review has identified that grade l or ll murmurs in newborns may be indicative of normal hearts, it is inadequately powered to state that with certainty. Therefore, practitioners must be mindful of two things. Firstly, the presence of murmurs in asymptomatic newborns greatly increases the chance of CHD. Secondly, since the DA sometimes doesn't close until after 48 hours, delaying discharge for 24 hours can at best only pick up a proportion of newborns with duct-dependent CCHD.
In this respect, the addition of pulse oximetry to the NPE is useful. Plana et al (2018) demonstrated that the sensitivity and specificity of this screen are unchanged by newborn age, other than the FPR, which is higher within the first 24 hours (0.42% vs 0.06%). The specificity of pulse oximetry is 99.9% (Plana et al, 2018). Therefore, the NPE practitioner examining within the first few hours after birth can, based on a negative pulse oximetry screen, be 99.9% sure that the newborn does not have CCHD.
It is important to recognise a continuum rather than a single point in time for CHD detection in newborns. This can be either through auscultation of murmurs or other signs and symptoms. This has significance for educating parents and midwives. Parents should be educated about the difficulties involved with screening for rare but potentially lethal forms of CCHD and informed of early signs and symptoms. An ideal time to impart that information is during the NPE. Midwives must be knowledgeable on this topic, as they visit newborns at home during the early days of neonatal life. In a study by Rogers et al (2015), only 13% of midwives were NPE-trained. However, this percentage will increase now that the skills and knowledge pertaining to the NPE are being incorporated into the preregistration midwifery curriculum (NMC, 2019).
Conclusion and practice recommendations
CHD is a significant cause of infant mortality and morbidity, meaning its detection is important. Newbor n screening for CHD at the NPE presents an opportunity to detect the clinically significant forms of the disease while babies are asymptomatic. This enables appropriate medical treatment and maximises the chances of good outcomes.
Based on the findings of this review, the author makes the following practice recommendations:
- The NPE should be conducted in a quiet, private room so that accurate auscultation is facilitated
- Experienced practitioners should be available to review all newborns with murmurs found at the NPE and enable the appropriate care pathway
- Pulse oximetry should be introduced as a universal screen for all newborns
- Echocardiography availability should be increased in UK hospitals so that CHD can be diagnosed or excluded promptly by diagnostic tests
- All parents should receive information at the time of the NPE on early signs and symptoms of significant CHD so that they can seek urgent medical assistance
- Given the lack of research in this area, studies should be conducted that include midwives' developing skills and knowledge in the art of auscultation and the detection of CHD.
Key points
- Auscultation of heart sounds at the newborn physical examination (NPE) forms a key part of newborn screening for congenital heart disease (CHD). However, a significant percentage of heart murmurs heard at the NPE relate to transitional physiology or clinically insignificant defects in the newborn and not to CHD
- There is significant heterogeneity found in the research relating to the statistics on prevalence of newborn murmur and its correlation to CHD. Nonetheless, the presence of heart murmur raises the background risk of cardiac malformation
- Detection of murmurs and hence detection of CHD improves with length of experience in the skill of auscultation, irrespective of professional background. Discerning accurately whether a murmur sounds innocent or pathological likewise improves with experience, and this can aid decision-making on the urgency of diagnostic echocardiography
- A large body of evidence supports the use of universal pulse oximetry as part of the NPE. This additional test has demonstrated a moderate sensitivity and a high specificity for the detection of rare but potentially lethal forms of critical CHD
CPD reflective questions
- When practising in a postnatal area, can you think of ways of increasing your skills in auscultation?
- Does pulse oximetry occur as part of newborn screening for congenital heart disease (CHD) in your hospital? If not, have a conversation with the neonatal lead at your unit and reflect on the complexity around decisions on screening policy
- When reviewing a newborn who is a few days old, do you consider the possibility of undiagnosed CHD? What signs and symptoms might you look for and what questions would you ask of the mother?


