Initiation of at-birth skin-to-skin contact and early breastfeeding is a well-established practice after vaginal birth (Moore et al, 2016). Over recent years, this practice is increasingly being implemented during caesarean section birth (Phillips, 2013). As a result of the increased frequency of maternal and neonatal hypothermia during caesarean section, this patient population are at an increased risk of health complications associated with hypothermia (Sultan et al, 2015). There is also a higher chance of maternal/newborn separation because of the need to manage postoperative hypothermia (Vilinsky and McCaul, 2017). Preventing perioperative neonatal and maternal hypothermia is key not only in supporting the mother–newborn dyad but also in promoting early and longer duration of skin-to-skin contact and breastfeeding during and after caesarean section (Vilinsky et al, 2016). The use of perioperative warm intravenous fluids is a well-established practice used in the prevention of inadvertent perioperative hypothermia in the general population undergoing an operation (Munday et al, 2014; Sultan et al, 2015). However, a systematic review found that it was not widely researched in pregnant women undergoing caesarean section while performing at-birth skin-to-skin contact, highlighting the need for more robust randomised controlled trials (Vilinsky-Redmond et al, 2020).
Aim
This study (the NeoHyp trial) compared the effectiveness of perioperative active warming by administering warmed intravenous fluids to women undergoing elective caesarean section and performing skin-to-skin contact at term, versus room temperature intravenous fluids, on neonatal and maternal outcomes.
Methods
Study design
This pragmatic double-blind parallel randomised controlled trial took place at an urban maternity hospital in Ireland from January to March 2018. The allocation sequence generated by the hospital statistician, with the use of computer randomisation software, allocated participants to either the intervention or control group. Allocation assignment was done using sequenced opaque sealed numbered envelopes generated by a midwife who was independent from the research team.
A midwife assessed participants' eligibility during their hospital admission, obtained written consent and collected baseline data, while the theatre anaesthetists provided allocated interventions. Participants were eligible to be part of the trial if they were aged 18 years or over, able to provide informed consent for themselves and their newborn, had a singleton pregnancy between 37+0 and 41+6 weeks gestation, their fetus/newborn was alive and healthy, received spinal or combined spinal anaesthesia for their caesarean section, had an elective caesarean section and were able to understand and communicate in English.
Sample size and participants
Based on a pilot randomised controlled trial (Vilinsky et al, 2016), SPSS software (version 25) calculated a measure of effect size (Cramer's V= 0.25). G*Power software estimated the sample size based on this effect size, an alpha level of 0.05, a power of 80% and df=1. The sample size was 126 participants, 63 women per group. Accounting for 20% attrition, the total sample size was estimated at 150 participants, 75 women per group.
In total, 150 of the 159 eligible patients consented and entered the trial (94.3% response rate and 5.6% refusal rate), with 75 women randomly assigned to each group (Figure 1).
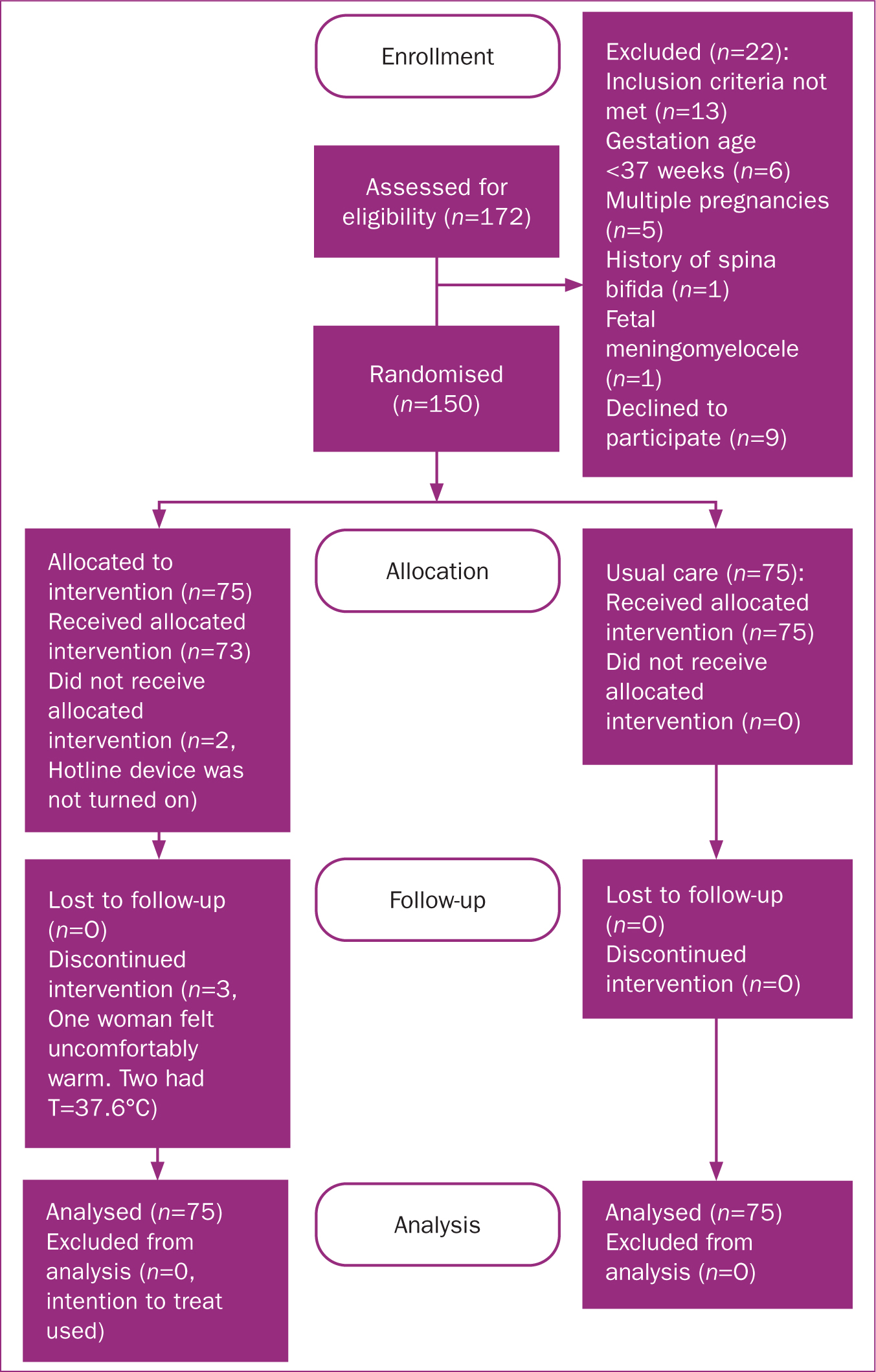
Measures
The primary outcome measure was neonatal hypothermia, assessed via axillar temperature measurements. Secondary outcomes included maternal hypothermia, shivering, thermal comfort, interruption of breastfeeding and skin-to-skin contact and neonatal and maternal adverse effects. Demographic and baseline data were collected at hospital admission, during caesarean section and in the post-anaesthesia care unit.
Trial intervention/control
Participants randomised to the intervention group received warm intravenous fluids (39°C) consisting of Hartman's solution with the use of the theatre's Hotline™ device. On arrival to the theatre, once the anaesthetist confirmed that a participant was allocated the intervention, they connected an intravenous Hartman's solution bag using the Hotline™ device. Intravenous administration commenced before administration of regional anaesthesia and was continued during the operation. Any additional intravenous fluids (ie antibiotics, paracetamol) were given at room temperature.
Participants randomised to the control group received the current standard of care in the hospital, perioperative administration of room temperature intravenous fluids (25°C) consisting of Hartman's solution initiated prior to the insertion of regional anaesthesia and administered until the woman was discharged from the post-anaesthesia care unit. On arrival to theatre, following confirmation that the participant was allocated to the control group, the anaesthetist connected a room temperature intravenous Hartman's solution bag to the Hotline™ device, which was not turned on, blinding participants and theatre staff to the assigned intervention. All bags of fluid given to patients were attached to the drip stand in theatre. The fluid warmer was attached to the bottom of the drip stand among other theatre devices, meaning the devices were below eye level, especially when the patient was lying on the operating table. The patients were not be aware of which bags were administered and which device was for warming. There were no visible indicators to patients that the device was open or inactive at the time of the fluid administration. Any additional intravenous fluids (ie antibiotics, paracetamol) were also given at room temperature.
All participants performed a period of skin-to-skin contact with their newborns at birth. Newborns were placed for skin-to-skin contact immediately after being dried and checked by the theatre midwives. Skin-to-skin contact during caesarean section lasts until the operation is over. The newborn is then temporarily removed from skin-to-skin contact, for theatre staff to prepare the mother for post-anaesthesia care unit admission, where skin-to-skin contact is resumed and breastfeeding initiated.
Data analysis
Using intention-to-treat analysis, statistical tests were 2-tailed, with P<0.05 considered statistically significant. Categorical data (neonatal and maternal hypothermia, maternal shivering, additional warming, adverse events) were analysed using a Chi-squared test. Continuous data (neonatal and maternal temperatures, neonatal weight, gestational age, maternal age) were analysed using mean, standard deviation and interquartile range (where appropriate). The 2-sample independent t-test (parametric test) and where appropriate the Mann-Whitney U test (non-parametric test) were used to estimate differences in group means. Pearson's correlation (correlation coefficient) investigated the relationship between continuous variables. Logistic binary regression analysis examined the relationship between neonatal hypothermia and predictors of the likelihood of neonatal hypothermia occurring.
Ethical considerations
Approval was granted by the Research Ethics Committee of the hospital in which the trial took place. Women gave informed consent for themselves and their newborns to take part in the study.
Results
Baseline characteristics
The majority of participants were over the age of 35 years (53%). Baseline characteristics (Table 1) were comparable overall between the two groups. Given the robust randomisation methods used in the trial, the imbalance in gestational age between the groups can be attributed to chance through randomisation.
Table 1. Baseline characteristics for intervention and control group participants
| Characteristic | Mean (standard deviation) | P value (t-test between groups) | ||
|---|---|---|---|---|
| Warm fluids, n=75 | Room temperature fluids, n=75 | Overall total | ||
| Maternal age (years) | 35.5 (5.51) | 34.4 (5.14) | 34.95 (5.32) | 0.46 |
| Gestational age (weeks) | 38.95 (0.93) | 38.5 (4.24) | 38.72 (2.58) | 0.03 |
| Birth weight (g) | 3470 (486.48) | 3479.5 (540.86) | 3474 (513.67) | 0.44 |
| Pre-theatre admission temperature of women (°C) | 36.45 (0.29) | 36.56 (0.34) | 36.48 (0.31) | 0.26 |
| Operating theatre ambient temperature (°C) | 23.87 (1.75) | 23.73 (1.30) | 23.8 (1.52) | .65 |
| Post-anaesthesia care unit ambient temperature (°C) | 23.73 (0.57) | 24.01 (0.84) | 23.87 (0.7) | .73 |
Hypothermia and thermal comfort
Categorical outcomes for mothers and newborns are shown in Table 2. Continuous outcomes are shown in Table 3. Perioperative administration of warm intravenous fluids was more effective in reducing the occurrence of neonatal hypothermia (defined as a temperature <36.5°C) at the end of skin-to-skin contact in the operative theatre than the standard practice of administrating room temperature intravenous fluids (P=0.02). But this difference was not evident at the time of post-anaesthesia care unit discharge (P=0.3).
Table 2. Neonatal and maternal categorical outcomes
| Outcome | Warm IV fluids, n=75 (%) | Room temperature fluids, n=75 (%) | P value | Relative risk (95% confidence interval) | Total, n=150 (%) |
|---|---|---|---|---|---|
| Neonatal hypothermia at end of skin-to-skin contact in operating theatre | 4 (5.3) | 14 (18.7) | 0.02 | 0.28 (0.09–0.82) | 18 |
| Neonatal hypothermia before post-anaesthesia care unit discharge | 0 (0) | 1 (1.5) | 0.30 | 0.33 (0.01–8.05) | 1 |
| Maternal hypothermia during skin-to-skin contact in the operating theatre | 2 (2.6) | 13 (17.3) | 0.0027 | 0.15 (0.03–0.65) | 15 |
| Maternal hypothermia on post-anaesthesia care unit admission | 10 (13.3) | 26 (34.6) | 0.0022 | 0.38 (0.2–0.74) | 36 |
| Maternal hypothermia on post-anaesthesia care unit discharge | 0 (0) | 5 (6.6) | 0.10 | 0.09 (0.005–1.61) | 5 |
| Maternal shivering | 3 (4) | 30 (40.0) | 0.0001 | 0.1 (0.03–0.31) | 33 |
| Additional maternal warming | 13 (17.3) | 42 (56.0) | 0.0001 | 0.31 (0.18–0.52) | 55 |
| Additional newborn warming | 8 (10.6) | 27 (36.0) | 0.0009 | 0.29 (0.14–0.6) | 35 |
| Maternal thermal comfort | 34 (45.3) | 33 (44.0) | 0.86 | 1.03 (0.72–1.47) | 67 |
| Newborn adverse events | 1 (1.3) | 0 (0) | 0.49 | 3 (0.12–72.48) | 1 |
| Maternal adverse events | 3 (4.0) | 0 (0) | 0.19 | 7 (0.36–133.22) | 3 |
| Interruption to skin-to-skin contact | 8 (10.6) | 27 (36.0) | 0.0009 | 0.29 (0.14–0.6) | 35 |
| Interruption to breastfeeding | 5 (6.6) | 17 (22.6) | 0.0076 | 0.27 0.10–0.71) | 22 |
| Breastfeeding | 53 (70.6) | 53 (70.6) | 1.0 | 1 (0.81–1.22 | 106 |
| Maternal complications | 2 (2.6) | 6 (8) | 0.16 | 3.3 (0.06–1.59) | 8 |
| Neonatal complications | 4 (5.3) | 5 (6.6) | 0.73 | 0.8 (0.22–2.86) | 9 |
Table 3. Neonatal and maternal continuous outcomes
| Outcome | Mean (standard deviation) | P value | ||
|---|---|---|---|---|
| Warm fluids, n=75 | Room temperature fluids, n=75 | Overall total | ||
| Maternal tympanic temperature during skin-to-skin contact in the operating theatre | 36.42 (0.44) | 36.48 (0.43) | 36.45 (0.43) | <0.00* |
| Maternal tympanic temperature on post-anaesthesia care unit admission | 36.27 (0.42) | 36.27 (0.45) | 36.27 (0.43) | 1.00† |
| Maternal tympanic temperature on post-anaesthesia care unit discharge | 36.54 (0.33) | 36.39 (0.36) | 36.46 (0.34) | 0.35† |
| Neonatal axillar temperature at the end of skin-to-skin contact in the operating theatre | 36.84 (0.34) | 36.89 (0.24) | 36.86 (0.29) | 0.02* |
| Neonatal axillar temperature on post-anaesthesia care unit discharge | 36.9 (0.21) | 36.95 (0.27) | 36.92 (0.24) | 1.18* |
| Mean skin-to-skin contact duration | 22 (8.84) | 24.33 (10.90) | 23.16 (9.87) | 0.81† |
| Mean total intravenous fluid volume | 1092.46 (532.26) | 1240.13 (473.42) | 1166.29 (502.84) | 0.51* |
| Mean estimated blood loss | 444.52 (240.75) | 473.02 (203.05) | 458.77 (221.9) | 0.20* |
t-test
Maternal hypothermia (defined as a temperature <36°C), when assessed during skin-to-skin contact in the operative theatre and on post-anaesthesia care unit admission occurred significantly less frequently in women receiving warmed intravenous fluids than those receiving room temperature intravenous fluids (P=.0027 and P=0.0022 respectively).
Women and neonates in the warm intravenous fluids group were warmer, with a significant difference in material mean tympanic temperature during skin-to-skin contact in the operating theatre and in neonatal mean axillary temperature at the end of skin-to-skin contact in the operating theatre compared to those receiving room temperature intravenous fluids (Table 3). Consequently, maternal shivering was significantly reduced in women receiving warm intravenous fluids (P=0.0001) and additional warming of mothers and newborns was required less frequently (P=0.0001 and P=0.0009 respectively).
Other neonatal and maternal outcomes
Most participants (89%) who received warm intravenous fluids experienced no interruption to skin-to-skin contact or breastfeeding (93%) with both groups having a comparable proportion of participants breastfeeding their newborns (53%) (Table 2). Furthermore, there were no differences in neonatal or maternal adverse events between mothers who received warm or room temperature intravenous fluids (P=0.49 and P=0.19 respectively).
There was no correlation between neonatal hypothermia and maternal temperature, ambient temperatures, duration of skin-to-skin contact, type of feeding, infant weight, intravenous fluid volume, estimated blood loss, gestational age or additional maternal/neonatal warming in either the operating room or post-anaesthesia care unit (Table 4).
Table 4. Logistic regression analysis of neonatal hypothermia
| Predictor | B coefficient | Standard error | P value (t-test) | Exponentiation of B coefficient | Odds risk (95% confidence interval) |
|---|---|---|---|---|---|
| Neonatal hypothermia at end of skin-to-skin contact in operating theatre | |||||
| Maternal temperature (during skin-to-skin contact in operating theatre) | 0.62 | 1.08 | 0.37 | 2.61 | 0.11 (0.31–22.14) |
| Maternal hypothermia (during skin-to-skin contact in operating theatre) | -0.39 | 0.95 | 0.68 | 0.67 | 3.75 (0.1–4.37) |
| Operating theatre ambient temperature | 0.02 | 0.2 | 0.9 | 1.02 | 0.82 (0.68–1.54) |
| Skin-to-skin contact duration | 0.06 | 0.03 | 0.9 | 1.06 | 0.97 (0.98–1.14) |
| Infants weight | 0.00 | 0.00 | 0.99 | 1.00 | 0.99 (0.99–1.00) |
| Additional intravenous fluid volume | 0.00 | 0.00 | 0.54 | 1.00 | 0.99 (0.99–1.00) |
| EBL | 0.00 | 0.00 | 0.98 | 1.00 | 0.99 (0.99–1.00) |
| Gestational age | 0.32 | 0.38 | 0.69 | 1.38 | 1.32 (0.64–2.65) |
| Maternal temperature (during skin-to-skin contact in operating theatre) | 0.62 | 1.08 | 0.37 | 2.61 | 0.11 (0.31–22.14) |
| Neonatal hypothermia before post-anaesthesia discharge | |||||
| Post-anaesthesia care unit ambient temperature | -57.19 | 1.18 | 0.07 | 2.11 | 8.25 (0.80–84.44) |
| Skin-to-skin contact duration | -8.23 | 0.09 | 0.23 | 0.00 | 1.11 (0.92–1.34) |
| Type of feeding: breastfeeding | 17.84 | 7154.11 | 0.99 | 0.00 | 37629.64 (0.00–n/a) |
| Type of feeding: formula | -13.63 | 7154.11 | 0.99 | 0.00 | 0.00 (0.00–n/a) |
| Additional maternal warming | -17.96 | 6325.70 | 0.99 | 0.00 | 0.00 (0.00–n/a) |
| Additional neonatal warming | -17.77 | 7929.68 | 0.99 | 79395132.07 | 0.00 (0.00–n/a) |
Discussion
To the best of the authors' knowledge, this study is the first known randomised controlled trial to focus on skin-to-skin contact in women undergoing caesarean section while exploring the effectiveness of perioperative maternal administration of warm intravenous fluids for the prevention of neonatal hypothermia. The use of warm intravenous fluids had several health benefits for both newborns and mothers, when compared to the comparator group. For participants receiving warm intravenous fluids, there was a significant reduction in neonatal hypothermia at the end of skin-to-skin contact in the operating theatre. Similar findings were reported in Vilinsky-Redmond et al's (2020) meta-analysis that included three randomised controlled trials investigating active warming devices during elective caesarean section; air warming devices (Horn et al, 2014) and warm intravenous fluids (Paris et al, 2014; Vilinsky et al, 2016). Paris et al (2014) investigated warm intravenous fluids in a three-arm trial but only measured neonatal temperature once, immediately after birth and before the initiation of skin-to-skin contact, with the primary focus on maternal hypothermia. The other two studies (Horn et al, 2014; Vilisnky et al, 2016) did not measure the effect of warm intravenous fluids on neonatal hypothermia during the newborns' stay in the post-anaesthesia care unit.
The present study is the only trial to explore the effect of active warming on newborns while undertaking skin-to-skin contact in the post-anaesthesia care unit, and found no difference between groups in the occurrence of neonatal hypothermia in the post-anaesthesia care unit, as all hypothermic newborns were actively warmed under the resuscitaire prior to their discharge from the post-anaesthesia care unit.
The present study found the frequency of maternal hypothermia during skin-to-skin contact in the operating theatre (P=0.0027) and on post-anaesthesia care unit admission (P=0.0022) to be significantly reduced in participants who received warm intravenous fluids, consistent with previous meta-analyses (Munday et al, 2014; Sultan et al, 2015; Vilinsky-Redmond et al, 2020). However, there is insufficient evidence from the wider literature (Vilinsky-Redmond et al, 2020) regarding the duration of maternal active warming and its effect on mothers.
A strength of the present study is the effects of active warming on maternal hypothermia were measured from the immediate period after caesarean section, both within and on discharge from the post-anaesthesia care unit. It is during the post-anaesthesia care unit stay that many early postoperative complications occur (Eichenberger et al, 2011) and therefore it is important to investigate the effect of maternal active warming on early postoperative complications, specifically on women after an elective caesarean section and performing skin-to-skin contact, adding to the knowledge base in this field.
This trial also showed a significantly lower use of additional warming for mothers (P=0.0001) who received warm intravenous fluids, similar to that found in a previous systematic review (Sultan et al, 2015). However, no other studies investigated the need for additional neonatal warming. This NeoHyp study, the first randomised controlled trial to investigate such an outcome, found that the use of neonatal additional warming was significantly lower in newborns whose mothers received warm intravenous fluids (P=0.0009).
A review of the literature found that no other studies investigated the effect of perioperative maternal active warming on interruption to skin-to-skin contact and breastfeeding. The present study showed significant lower interruption to skin-to-skin contact and breastfeeding in the intervention group. Reducing maternal/newborn separation supports theatre staff to promote skin-to-skin contact within the theatre department and initiate early breastfeeding (Moore et al, 2016). Increasing the initiation and duration of skin-to-skin contact and breastfeeding has significant health benefits for both mothers and newborns (Boundy et al, 2016; Moore et al, 2016).
Another strength of the present study is that it monitored and documented adverse events that occurred in both mothers and newborns. Overall, there was no significant difference in the occurrence of maternal (P=0.19) and neonatal (P=0.49) adverse events between the two groups. This suggests that administration of warm intravenous fluids is a safe intervention for both mothers and their newborns. It is important to monitor maternal temperatures during caesarean section; any increase in maternal temperature above recommended levels can be managed effectively with discontinuation of the intervention.
There is evidence that delayed cord clamping can increase neonatal temperature, as transfused blood received by the baby is warmer than neonatal temperature, which could influence the trajectory of the neonatal temperature. However, delayed cord clamping during caesarean section was not a current practice in the theatre at the time that the study was conducted. All cords were clamped after the birth of the baby and babies were transferred to the resuscitate before skin-to-skin contact was initiated. A future study could examine the effect of delayed cord clamping on babies' temperatures when mothers receive warmed fluids.
The present study is the first trial to run a logistic regression model to identify predictive factors for neonatal hypothermia other than the intervention itself. The regression analysis did not find any other predictive variables. One could assume that maternal administration of warm intravenous fluids is the only predictive factor in preventing neonatal hypothermia during elective caesarean section, while at birth skin-to-skin contact is performed.
Limitations
At the time of undertaking the trial, a new electronic chart system was introduced to the hospital. As a result, some outcome data were missing, including 18 records of neonatal hypothermia (before post-anaesthesia care unit discharge) and 11 of maternal hypothermia (during skin-to-skin contact in the operating theatre). This may have occurred as a result of errors in operating the new chart system or errors in inputting the data by staff. The problem of missing data was anticipated and was managed using intention to treat during the data analysis phase. Furthermore, a sensitivity analysis was carried out to assess the potential impact of neonatal and maternal hypothermia on participants whose data were not documented, comparing worst vs best case scenarios. The sensitivity analysis suggested that the results of both the primary and secondary outcomes were robust, something that illustrates additional rigour to the trial.
Women who required emergency caesarean section were excluded. Therefore, this study was unable to investigate the effect of the intervention on this cohort of people. A final limitation is that this was not a multicentre study, but rather undertaken in one large urban maternity hospital. Single centre trials have the potential not to recruite a sufficient number of participants; however, the sample size of the trial was carefully estimated to be representative of the population before the trial was conducted and the hospital annual elective caesarean section rate was sufficient to allow the sample size to be recruited within the time limits agreed in the trial protocol.
Conclusions
This is the first study to prove that administration of warm intravenous fluids on pregnant women undergoing elective caesarean section is a safe practice for both mothers and newborns, which would allow for the performance of uninterrupted at-birth skin-to-skin contact and early initiation of breastfeeding within theatre departments. The study's contribution to the evidence highlights the positive impact an active warming intervention has in women undergoing an elective caesarean section while at-birth skin-to-skin contact is performed. It reduces the occurrence of maternal and neonatal hypothermia, reducing the need for additional maternal or neonatal warming, which in turn reduces the need for maternal–newborn separation and allows for uninterrupted at birth skin-to-skin contact and early breastfeeding to take place immediately after an elective caesarean section.
Key points
- This is the first randomised controlled trial exploring the effectiveness of perioperative maternal administration of warm intravenous fluids for the prevention of neonatal hypothermia.
- Neonatal hypothermia at the end of skin-to-skin contact in the operating theatre can be reduced with the use of maternal active warming.
- Perioperative maternal active warming reduces the incidence of maternal hypothermia during/after elective caesarean sestion.
- Perioperative maternal active warming does not increase a risk for maternal/neonatal adverse events.


