High body mass index (BMI) is currently the world's most common health problem. From 1980–2015, the prevalence of higher than normal BMI has doubled worldwide and has increased continuously in most countries. A total of 107.7 million children and 603.7 million adults are obese, which leads to an estimate of 30% of the world's population being affected by weight problems (GBD Obesity Collaborators, 2017).
There is also an alarming shift towards increasing weight gain among women of reproductive age. According to the National Survey of Family Growth (Vahratian, 2009) in the US, almost one-third of women of reproductive age (20–44 years) were overweight (BMI: 25.0–29.9 kg/m2) and 23.0% were obese (BMI≥30.0 kg/m2) in 2002. Additionally, the Pregnancy Risk Assessment Monitoring System data from nine states in the US (Kim et al, 2007) has shown that the incidence of obesity in early pregnancy has increased from 13% in 1994 to 22% in 2003. Similarly, the National Health Survey in Australia has reported that 24% of all Australian women aged 25–44 years old were overweight, and 14% were obese, in 2004–2005 (Australian Bureau of Statistics, 2008). Unlike these reports, research by McIntyre et al (2012) showed a lack of a marked temporal trend towards increasing maternal obesity in pregnant women in Australia between 1998–2009.
The growing burden of high BMI among pregnant women has been suggested to have substantial national health consequences and is associated with both short- and long-term health effects for women and their offspring (Kim et al, 2007). It is linked to perinatal complications, such as infertility, miscarriage, gestational hypertension, gestational diabetes, pre-term labour, caesarean section and fetal macrosomia, congenital anomalies and death (Santangeli et al, 2015). Children of mothers with a high BMI at the beginning of pregnancy were 2.3 times more likely to be obese at 4 years old (Whitaker, 2004) and have a greater risk of metabolic syndrome and obesity at 11 years (Boney et al, 2005). An Australian study (McIntyre et al, 2012) has shown an association between high maternal BMI and a broad spectrum of adverse maternal and neonatal pregnancy outcomes.
A systematic review of the literature showed a gap in the literature and indicated that there is a lack of current data on the limited research on the time-trends of BMI in early pregnancy and the effect of the trends on pregnancies in women with high BMI, considering their demographics and medical changes over the years.
Study objectives
The primary objective of this study was to explore changes in the prevalence and trends of early pregnancy BMI during 2011–2017 among pregnant women who received antenatal care and gave birth in a tertiary hospital in western Sydney, New South Wales. The secondary objectives were to identify the risk factors of high BMI from 2011–2017 and to explore trends in the nominated maternal and neonatal outcomes from 2011–2017 in relation to high BMI and other confounding factors.
Methods
Design and setting
This retrospective, cohort study was conducted at a tertiary hospital in Sydney that is one of the largest clinical settings in Australia and provides maternity care to more than 5 500 women per year.
Data source
The electronic maternity database ObstetriX was used to identify records of all women who gave birth at the hospital between January 2011 and December 2017. The ObstetriX database contains perinatal information for every pregnancy and birth in most New South Wales hospitals.
Outcomes and covariates
Women's weight during early pregnancy (at the booking-in visit during the first trimester at 10–13 weeks) was categorised into underweight (BMI<18.5), healthy weight (BMI:18.5–24.9), overweight (BMI:25–29.9) and obese (BMI≥30). For the purpose of this paper, overweight and obese groups were reported together as ‘high BMI’ (BMI≥25) throughout.
Birth outcomes include gestational diabetes (including diet-controlled and insulin-controlled gestational diabetes, gestational hypertension (including pre-eclampsia and eclampsia), gestational age at birth <37 weeks, caesarean section, episiotomy, admission to the special care nursery or neonatal intensive care unit, postpartum haemorrhage, and neonatal birth weight.
In addition to obesity and year, three sets of factors were examined where appropriate. First, demographic data, including age and country of birth. Second, maternal factors, including parity, type of conception and antenatal model of care (the doctor-led model of care included hospital-based medical obstetrician GP or GP-share care and obstetrician care (private or public)). The final factor was health status, including diabetes, endocrine diseases (including thyroid, adrenal and pituitary disease), renal diseases (including urinary tract infection, pyelonephritis, congenital renal disease, stress incontinence, lack of bladder sensation and glomerulonephritis), neurological diseases (including migraine, epilepsy, neuromuscular disease or neuripathy, multiple sclerosis, encephalitis or meningitis, benign intracranial hypertension and spinal cord disease), autoimmune diseases (including systemic lupus erythematosus, rheumatoid antibodies, antiphospholipid antibodies, connective tissue disease and other) and hypertension.
Data analysis
Excel was used to prepare and clean the data, which were transferred to R package, version 3.3.0 for statistical analyses (R Core Team, 2016). Descriptive statistics were used to summarise the demographic data and multivariable regression modelling was used to estimate adjusted effects of variables of interest. To calculate the relative risk (RR) of episiotomy and third and fourth degree tears, data for women who gave birth via caesarean section were excluded. To minimise the effect of other pre-existing conditions on birth outcomes, pre-existing hypertension, type I diabetes (73 women) and type II diabetes (231 women) were excluded from the regression analysis. Differences were considered statistically significant at P<0.05.
The models generated using multivariable regression modelling were adjusted for year, age, country of birth, parity, smoking and alcohol consumption, and medical conditions (including endocrine, renal, neurological and autoimmune diseases). Additional adjustments were made in the model for trends of perinatal outcomes among women with high BMI, the model for maternal and neonatal outcomes among women with high BMI, and the model for neonatal birth weight among women with high BMI. These adjustments were for high BMI, assisted conception (excluded in the analysis of assisted conception trend), antenatal model of care, medical conditions (including endocrine, renal, neurological and autoimmune diseases), gestational diabetes (excluded in the analysis of gestational diabetes trend), gestational hypertension (excluded in the analysis of gestational hypertension trend) and neonatal birth weight.
Results
A total of 38 857 pregnant women received antenatal care and gave birth at the hospital during 2011–2017. Removal of incomplete data resulted in 37 450 records used in data analysis. Of these, 5 633 women (86%) gave birth twice, 771 (12%) gave birth three times and 110 (2%) gave birth five or more times.
Table 1 shows data relating to the maternal characteristics of the study population. Of 37 450 women, 2 111 (5.6%) were underweight, 20 241 (54.0%) had a healthy weight, and 15 098 (40.3%) had a high BMI (24.1% of women were overweight and 16.2% were obese) during early pregnancy.
Table 1. Maternal characteristics (2011–2017)
| Variable | Underweight or normal (n=22 352) | Overweight or obese (n=15 098) |
|---|---|---|
| Maternal age>35 years | 3 645 (16.3%) | 3 319 (22.0%) |
| Country of birth: Australia 6 | 613 (29.6%) | 6 122 (40.5%) |
| Gestational age at birth<37 weeks | 1 501 (6.7%) | 1 220 (8.1%) |
| Parity: multiparous | 11 712 (52.4%) | 9 880 (65.4%) |
| Smoking during pregnancy: yes | 1 215 (5.4%) | 1 211 (8.0%) |
| Alcohol consumption during pregnancy: yes | 232 (1.0%) | 172 (1.1%) |
| Type of conception: assisted | 1 015 (4.5%) | 891 (5.9%) |
| Model of care during pregnancy: doctor-led | 11 704 (52.4%) | 9 537 (63.2%) |
| Endocrine disease: yes | 2 423 (10.8%) | 1 742 (11.5%) |
| Renal disease: yes | 2 049 (9.2%) | 1 562 (10.3%) |
| Neurological disease: yes | 1 771 (7.9%) | 1 591 (10.5%) |
| Auto-immune disease: yes | 311 (1.4%) | 227 (1.5%) |
| Gestational diabetes: yes | 2 648 (11.8%) | 1 856 (12.3%) |
| Gestational hypertension: yes | 1 024 (4.6%) | 732 (4.8%) |
Primary objective: trends of high BMI from 2011–2017
The rate of high BMI at early pregnancy increased from 37% in 2011 to 44% in 2017 (P<0.001) (Table 2; Figure 1). As shown in Table 3, the RR of high BMI in early pregnancy increased by an average of 3% annually.
Table 2. Early pregnancy maternal BMI (2011−2017*)
| Underweight or normal | Overweight or obese | Total | |||
|---|---|---|---|---|---|
| Year | (n=22 352) | (n=15 098) | (n=37 450) | ||
| 2011 | 3 353 | (63.0%) | 1 973 | (37.0%) | 5 326 |
| 2012 | 3 438 | (63.6%) | 1 966 | (36.4%) | 5 404 |
| 2013 | 3 276 | (61.4%) | 2 063 | (38.6%) | 5 339 |
| 2014 | 3 282 | (59.1%) | 2 269 | (40.9%) | 5 551 |
| 2015 | 3 160 | (57.7%) | 2 316 | (42.3%) | 5 476 |
| 2016 | 3 171 | (56.8%) | 2 412 | (43.2%) | 5 583 |
| 2017 | 2 672 | (56.0%) | 2 099 | (44.0%) | 4 771 |
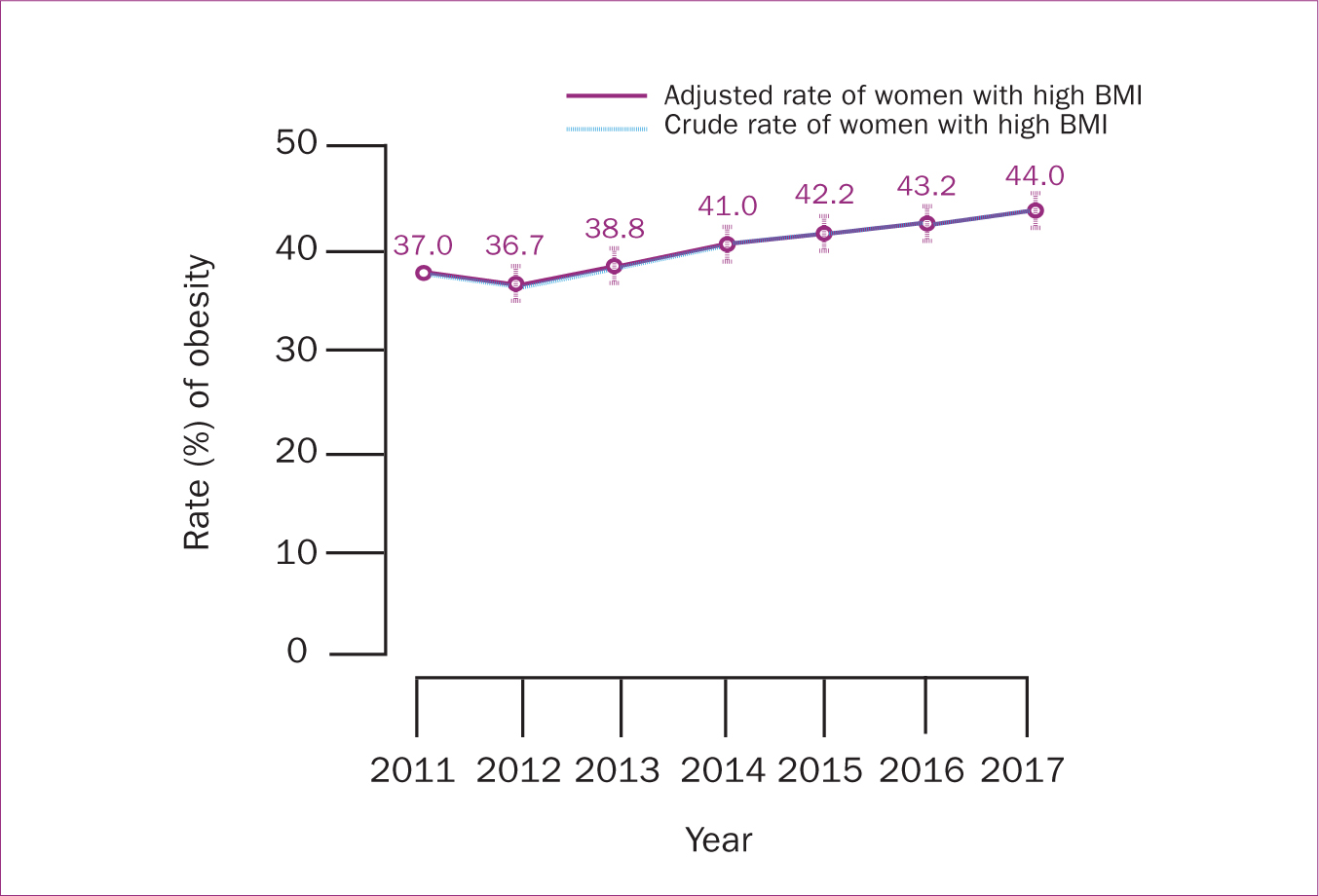 Figure 1. Trends of high BMI, including overweight and obese among the study population from 2011−2017 using multivariate regression modelling
Figure 1. Trends of high BMI, including overweight and obese among the study population from 2011−2017 using multivariate regression modelling
Table 3. Relative risk of high BMI, adjusted for maternal characteristics using multivariate regression modelling
| Characteristics | Overweight or obese relative risk (95% CI) |
|---|---|
| Year (incremental effect) | 1.03 (1.03−1.04)* |
| Age>35 years | 1.15 (1.11−1.20)* |
| Non-Australian born | 0.77 (0.75−0.80)* |
| Parous | 1.34 (1.30−1.39)* |
| Smoking | 1.11 (1.04−1.18)* |
| Alcohol consumer | 0.98 (0.84−1.14) |
| Endocrine disease | 1.03 (0.98−1.08) |
| Renal disease | 1.03 (0.98−1.09) |
| Neurological disease | 1.14 (1.08−1.20)* |
| Autoimmune disease | 0.95 (0.83−1.08) |
Secondary objective: adjusted relative risks of high BMI
The RR of high BMI was 15% greater in women who were more than 35 years old, 34% greater in multiparous women, 11% greater in women who were smoking, and 14% greater in women with neurological disorders (including migraine, epilepsy, benign intracranial hypertension, cerebrovascular and neuromuscular diseases, neuropathy, multiple sclerosis and spinal cord disease). The RR of high BMI was lower in women who were born outside Australia (P<0.05) (Table 3).
Secondary objective: adjusted relative risks and effect size of maternal and neonatal outcome
The risk of gestational diabetes, episiotomy, postpartum haemorrhage and smaller average birth weight increased significantly from 2011–2017 (P<0.05). However, the risks of assisted conception, gestational age<37 weeks, caesarean section, third and fourth degree tears, and neonatal admission to the special care nursery or neonatal intensive care unit were almost steady during the study period (P<0.05) (Table 4; Figures 2, 3,4).
Table 4. Adjusted relative risks and effect size of maternal and neonatal outcomes from 2011−2017 using multivariate regression modelling
| Relative risk (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Gestational diabetes | Gestational hypertension | Assisted conception | Gestational age at birth (<37 weeks) | Caesarean section | Episiotomy | 3rd and 4th degree tears | Special care nursery/neonatal intensive care unit | Postpartum haemorrhage | Neonatal birth weight (effect size) |
| Year (incremental effect) | 1.09 (1.08−1.11)** | 0.98 (0.95−1) | 1.01 (0.99−1.03) | 1.00 (0.98−1.02) | 1.01 (1−1.02) | 1.07 (1.06−1.08)** | 0.97 (0.93−1) | 1.01 (1−1.02) | 1.10 (1.07−1.13)** | -8.02 (-10.49−5.55) |
| Overweight or obese | 1.00 (0.94−1.06) | 1.05 (0.95−1.16) | 1.27 (1.15−1.39)** | 1.06 (0.98−1.14) | 1.28 (1.23−1.33)** | 0.84 (0.8−0.88)** | 0.90 (0.77−1.05) | 1.23 (1.16−1.3)** | 0.97 (0.87−1.07) | 129.16 (119.12−139.19) |
| Age>35 years | 1.00 (0.92−1.08) | 0.98 (0.86−1.11) | 2.96 (2.68−3.27)** | 1.24 (1.13−1.36)** | 1.31 (1.25−1.37)** | 1.08 (1.01−1.16)* | 0.94 (0.75−1.18) | 1.20 (1.12−1.28)** | 1.03 (0.9−1.18) | 10.11(-2.73−22.94) |
| Non-Australian born | 0.99 (0.93−1.06) | 0.96 (0.86−1.06) | 0.80 (0.73−0.88)** | 0.80 (0.74−0.87)** | 1.19 (1.14−1.24)** | 1.64 (1.55−1.73)** | 2.50 (2.08−3.01)** | 0.93 (0.88−0.99)* | 1.23 (1.1−1.37)** | -109.92 (-120.47− -99.37)** |
| Parous | 1.03 (0.97−1.1) | 1.07 (0.97−1.19) | 0.35 (0.31−0.38)** | 0.73 (0.68−0.8)** | 0.88 (0.84−0.92)** | 0.27 (0.25−0.28)** | 0.25 (0.21−0.3)** | 0.70 (0.66−0.73)** | 0.65 (0.59−0.72)** | 118.05 (107.94−128.16) |
| Smoking | 0.98 (0.87−1.12) | 0.99 (0.81−1.2) | 0.35 (0.26−0.48)** | 1.33 (1.16−1.53)** | 0.96 (0.88−1.04) | 0.64 (0.55−0.73)** | 0.37 (0.21−0.66)** | 1.16 (1.05−1.28)** | 1.03 (0.82−1.29) | -124.37 (-144.59–-104.14)** |
| Alcohol consumer | 0.98 (0.72−1.32) | 0.46 (0.24−0.9)* | 0.84 (0.53−1.34) | 1.19 (0.87−1.63) | 1.09 (0.92−1.3) | 1.21 (0.97−1.51) | 1.23 (0.61−2.48) | 1.07 (0.85−1.34) | 1.20 (0.75−1.91) | 25.76 (-21.02−72.55) |
| Assisted conception | 1.08 (0.95−1.24) | 0.88 (0.69−1.11) | 1.78 (1.57−2.01)** | 1.18 (1.09−1.27)** | 1.09 (0.99−1.21) | 1.20 (0.88−1.63) | 1.16 (1.06−1.28)** | 1.34 (1.09−1.64)** | -80.12 (-102.67–-57.57) | |
| Doctor-led antenatal model of care† | 1.06 (0.99−1.12) | 0.93 (0.85−1.03) | 2.01 (1.81−2.24)** | 4.08 (3.67−4.54)** | 1.81 (1.74−1.89)** | 1.17 (1.12−1.23)** | 0.96 (0.84−1.11) | 2.09 (1.95−2.23)** | 1.05 (0.94−1.16) | -93.41 (-103.45–-83.38) |
| Endocrine disease | 1.53 (1.41−1.65)** | 1.26 (1.1−1.45)** | 0.89 (0.77−1.04) | 1.02 (0.91−1.15) | 1.01 (0.95−1.07) | 0.97 (0.9−1.04) | 0.96 (0.77−1.2) | 1.02 (0.93−1.1) | 1.03 (0.88−1.2) | 4.03 (-11.34−19.4) |
| Renal disease | 0.99 (0.9−1.1) | 1.03 (0.88−1.2) | 0.87 (0.74−1.02) | 1.08 (0.95−1.22) | 1.03 (0.97−1.1) | 0.95 (0.88−1.03) | 0.81 (0.62−1.05) | 1.01 (0.93−1.1) | 1.07 (0.9−1.26) | 6.71 (-9.67−23.09) |
| Neurological disease | 1.04 (0.94−1.15) | 1.01 (0.85-1.19) | 1.03 (0.89−1.2) | 0.95 (0.83−1.08) | 1.08 (1.01-1.15)* | 1.00 (0.92−1.08) | 0.99 (0.76−1.28) | 1.11 (1.02−1.21)* | 1.10 (0.93−1.31) | -1.35 (-18.31−15.62) |
| Autoimmune disease | 1.07 (0.85−1.36) | 0.98 (0.65-1.47) | 1.16 (0.85−1.59) | 1.17 (0.9−1.51) | 1.01 (0.87−1.17) | 1.08 (0.89−1.3) | 1.36 (0.77−2.42) | 0.98 (0.8−1.19) | 1.05 (0.69−1.58) | -10.46 (-51.12−30.2) |
| Gestational hypertension | 1.23 (1.08−1.39)** | - | - | 1.08 (0.9−1.28) | 0.96 (0.88−1.05) | 1.01 (0.91−1.12) | 1.12 (0.82−1.53) | 0.95 (0.83−1.07) | 1.08 (0.85−1.36) | 5.50 (-17.27−28.28) |
| Gestational diabetes | - | 1.25 (1.1−1.43)** | - | 1.05 (0.93−1.18) | 1.06 (1-1.12)* | 1.02 (0.96−1.1) | 0.85 (0.68−1.07) | 0.99 (0.91−1.07) | 0.91 (0.78–1.07) | -6.16 (-21−8.68) |
| Birth weight (incremental effect) | - | - | - | - | 1.00 (1−1) | 1.00 (1−1) | 1.00 (1−1) | - | 1.00 (1−1) | - |
| Gestational age (<37 weeks) | - | - | - | - | 1.57 (1.46−1.69)** | 0.87 (0.77−0.99)* | 0.61 (0.36−1.04) | 5.20 (4.91−5.51)** | 1.75 (1.39−2.21)** | -1090.21 (-1109.11–- -1071.31)** |
significant at P<0.01
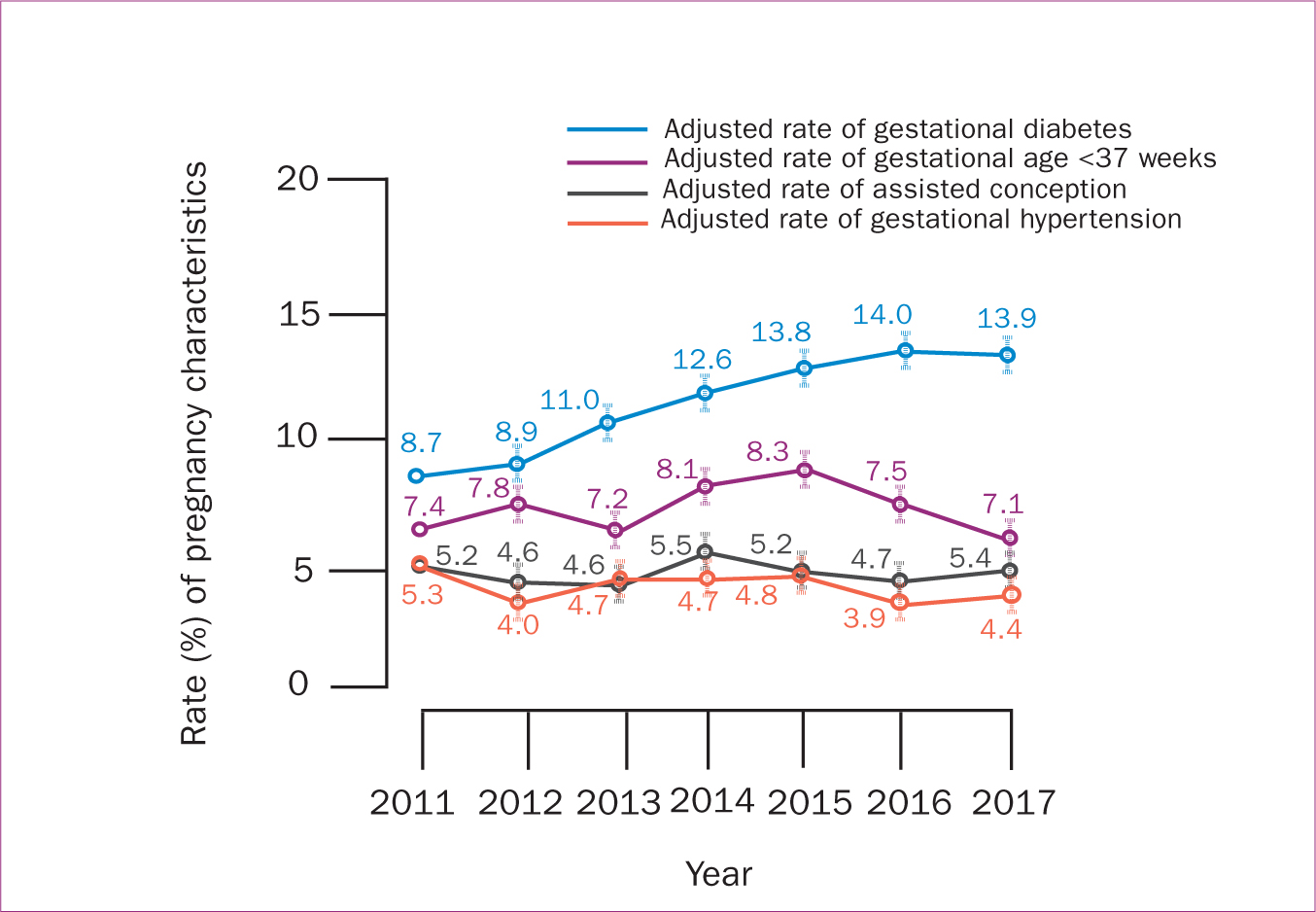 Figure 2. Trends of perinatal outcomes among women with high BMI including overweight and obese from 2011−2017 using multivariate regression modelling
Figure 2. Trends of perinatal outcomes among women with high BMI including overweight and obese from 2011−2017 using multivariate regression modelling 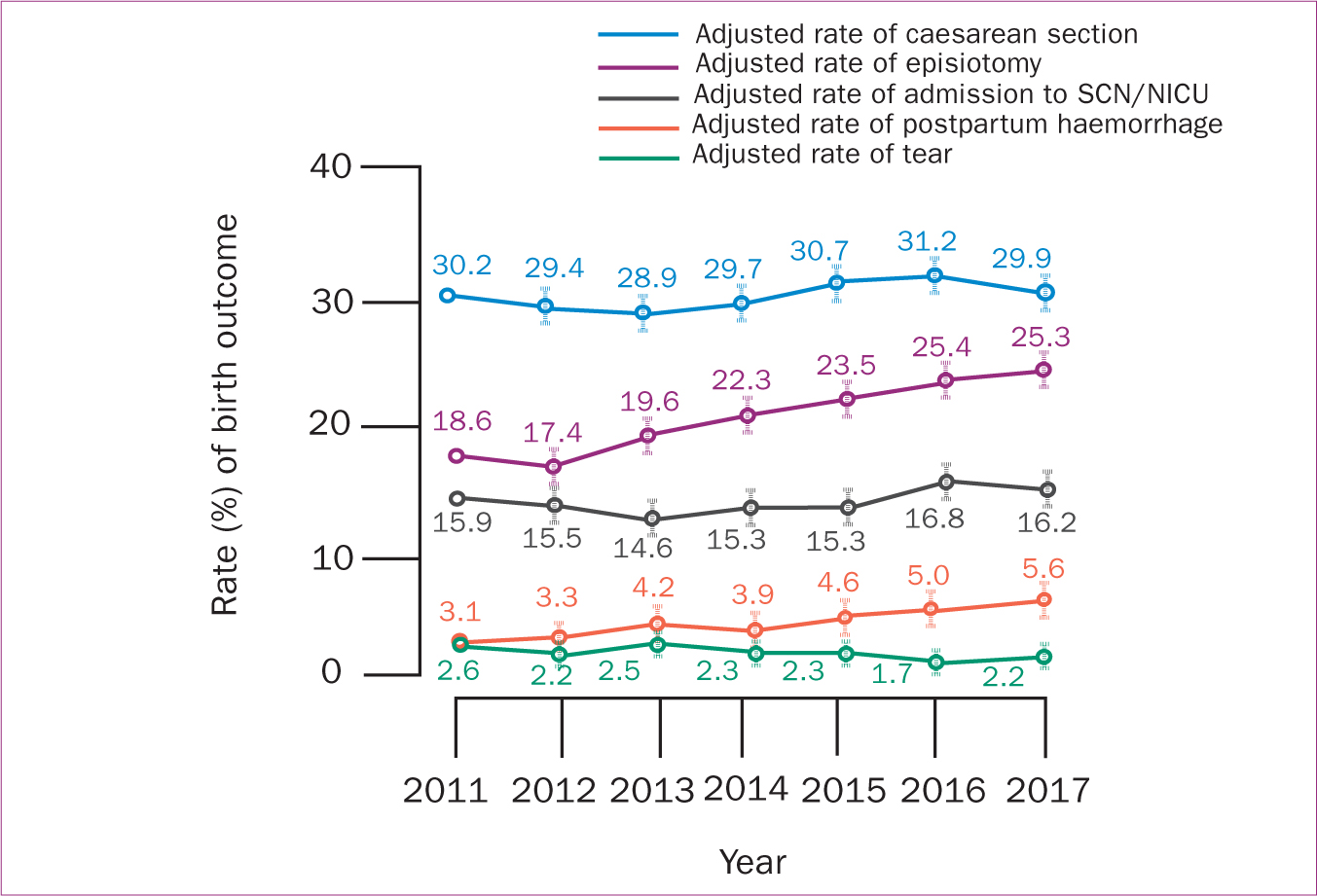 Figure 3. Trends of maternal and neonatal outcomes among women with high BMI including overweight and obese from 2011−2017 using multivariate regression modelling
Figure 3. Trends of maternal and neonatal outcomes among women with high BMI including overweight and obese from 2011−2017 using multivariate regression modelling 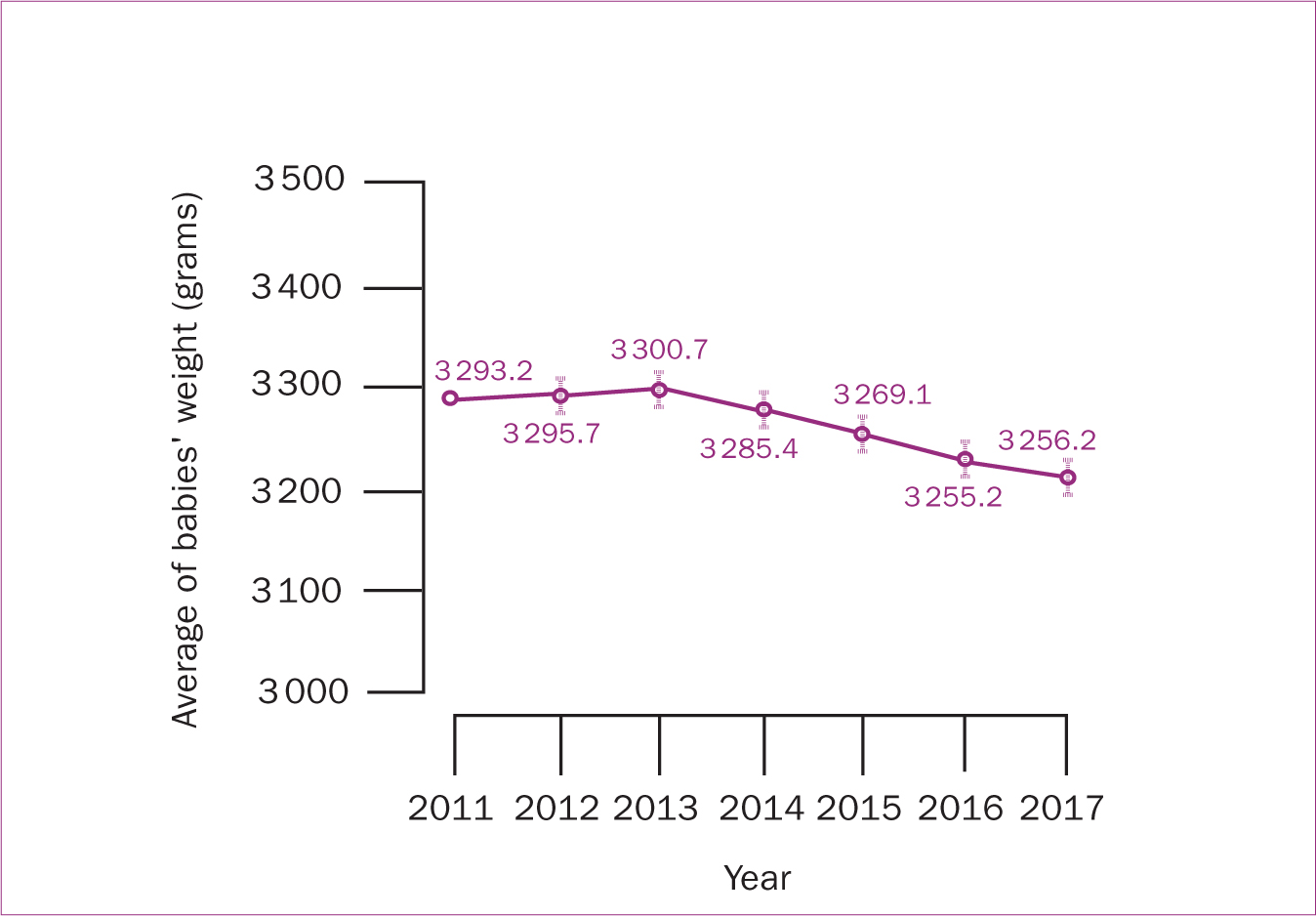 Figure 4. Trends of neonatal birth weight among women with high BMI (including overweight and obese) from 2011−2017 using multivariate regression modelling
Figure 4. Trends of neonatal birth weight among women with high BMI (including overweight and obese) from 2011−2017 using multivariate regression modelling
As shown in Table 4, to investigate the RR of overall untoward perinatal outcomes among women with high BMI, the model was adjusted for the year, high BMI, age, country of birth, parity, smoking and alcohol consumption, antenatal model of care, endocrine, renal, neurological and autoimmune disease, assisted conception, gestational diabetes, gestational hypertension, gestational age at birth and neonatal birth weight (the latter five variables were both outcomes and predictors).
The regression model showed that the RR of assisted conception was greater in women with high BMI and women who were >35 years old and had a doctor-led antenatal model of care (P<0.05). The RR of giving birth at <37 weeks of gestation was greater in women who were >35 years old, were smoking, had assisted conception and had a doctor-led model of care. However, the RR was lower in non-Australian women and multiparous women (P<0.05) (Table 4).
The RR of having a caesarean section was greater in women who had a high BMI, were >35 years old, were non-Australian, had assisted conception, had a doctorled antenatal model of care, had any type of neurological disease, had gestational diabetes and who gave birth at gestational age <37 weeks. The RR of caesarean section was lower in multiparous women (P<0.05) (Table 4).
The overall risk of episiotomy was higher in women more than 35 years old, were non-Australian, had a doctor-led antenatal model of care and had larger neonates. The RR of episiotomy was lower in women who had a high BMI, were multiparous and in women who were smoking during pregnancy (P<0.05). While women who were non-Australian born were more likely to have third and fourth degree tears, the risk was lower in parous women and those who were smoking during pregnancy (Table 4).
Neonates were more likely to be admitted to the special care nursery or neonatal intensive care unit if they were born at gestational age <37 weeks and their mothers had a high BMI, were aged >35 years old, had assisted conception, smoked during pregnancy, received doctor-led antenatal care and had a neurological disease (P<0.05). A high BMI did not affect the RR of postpartum haemorrhage. However, being non-Australian born, having assisted conception and giving birth at gestational age <37 weeks were significant risk factors for postpartum haemorrhage, while the RR was lower in parous women (P<0.05) (Table 4).
Neonates of women with a high BMI and multiparous women were 129 g and 118 g, respectively, larger than neonates of women with a normal BMI or underweight, and nulliparous women (P<0.05). Neonates of non-Australian born women and women who were smoking, had assisted conception, a doctor-led antenatal model of care, and gave birth before 37 weeks of gestation were more likely to be smaller than neonates of other women (Table 4).
Discussion
The rate of high BMI during early pregnancy increased by approximately 3% annually and rose from 37% in 2011 to 44% in 2017. After adjusting for potential risk factors, high BMI was shown to be associated with maternal age >37 years, multiparity, smoking and neurological disease (opposing some of the unadjusted findings). The adjustment eliminated confounding effects, reduced the variability between the subjects and provided isolated and true effects of the risk factors. Women with high BMI who were born in countries other than Australia were from America (1%), Asian countries (33%), European countries (2%), North Africa and Middle East (13%), Sub-Saharan (3%), and Oceania and Antarctica (46%), with 2% unknown. Overall, high maternal BMI was associated with greater risks of having an assisted conception, a caesarean section and larger neonates, and smaller risks of episiotomy and neonatal admission to the special care nursery or neonatal intensive care unit.
These findings agree with international data from 184 countries that show an increase in maternal obesity in high- and middle-income countries between 2005–2014 (Chen et al, 2018). According to pre-pregnancy BMI data from 48 states in the US (Deputy et al, 2018), the prevalence of pre-pregnancy normal weight decreased by 5% from 2011–2015, whereas the prevalence of being overweight increased by 2%, and the prevalence of all classes of obesity increased by 8%. In 2015, just over one-quarter (25.6%) of American pregnant women were obese (Deputy et al, 2018). A UK study also reported an increasing rate from 7.6% in 1989 to 17.6% in 2007 (Heslehurst et al, 2010).
The present study's findings, and those of previous research indicate that society is shifting away from the 2020 Healthy People target for pre-pregnancy normal weight. A high BMI in early pregnancy was associated with older age (>35 years), parity, smoking and chronic neurological disease. These findings support previous research suggesting a high BMI at early pregnancy is associated with risk factors that are mostly not modifiable before pregnancy, such as age, parity and chronic disease (Reynolds et al, 2019). Nevertheless, justification for the increased maternal weight from 2011–2017 in this study is challenging. The study period was too short (7 years) for genetics to be an explanation. Also, the non-Australian-born trend was steady over the study period, and the risk of high BMI was significantly smaller in non-Australian-born women, so the upward trend cannot be related to demographic changes or migration. Since this was a retrospective study, there was no data on other confounding factors, such as economic status of the family, lifestyle, physical activity, duration of residency in Australia (for non-Australia-born women), intake of unhealthy convenience foods, etc. The authors believe it is fair to assume that the increasing rate of high BMI might be the result of changes in social factors and lifestyle changes, as suggested by Rosengren et al (2008).
The larger birth weight in neonates of women with a high BMI in the present study agrees with earlier research that showed an increase in the frequency of macrosomia with the increase of maternal weight (Breckenkamp et al, 2019). The greater risk of caesarean section in women with a high BMI has been shown to be the result of the increasing risk of induction of labour, which is more likely to be followed by caesarean section, because of concerns about shoulder dystocia (Wispelwey and Sheiner, 2013). Nevertheless, these findings are in contrast with that of other research indicating that lower birth weight is more common among babies of obese women because they are more likely to have labour induced early or undergo a caesarean section at an earlier gestational age than women with a normal BMI. Therefore, their neonates are more likely to be smaller for reasons such as a higher risk of gestational hypertension or gestational diabetes (Cody et al, 2016).
In the present study, high BMI decreased the risk of episiotomy and did not affect the risk of third and fourth degree tears. Indeed, while statistically non-significant, the risk of third and fourth degree tears was lower in women with high BMI (RR=0.90; 95% CI=0.77–1.05). This finding is in accordance with that of earlier studies indicating that obesity plays a protective role against anal laceration (Lindholm and Altman, 2013, Garretto et al, 2016). While there is limited research on the impact of high BMI on perineal and anal lacerations, the following theories are suggested to explain the protective role of obesity: women with high BMI may have different composition of the perineal tissue, which in turn facilitates perineal stretching during labour and birth (Hirayama et al, 2012); high BMI may interfere with and decrease the force and frequency of uterine contractions and may minimise subsequent pelvic floor injuries (Zhang et al, 2007). The importance of avoiding third and fourth degree tears is not only because of the inflicted pain and discomfort but also the potential association between these advanced tears and bowel incontinence (Sideris et al, 2020). Even after proper surgical repair of third and fourth degree tears, symptoms of bowel incontinence are reported in many women, which in turn affects their health, wellbeing and self-image (Hirayama et al, 2012; Sideris et al, 2020). This suggests that the pelvic floor trauma resulting in bowel incontinence may not be as likely to occur because of birth trauma. Indeed, it may come from the enhanced intra-abdominal pressure, life style, dietary habits and other concomitant risk factors in women with high BMI (Garretto et al, 2016).
Study limitations and strengths
This study has some inherent limitations because of its retrospective nature. Although the distribution of BMI among the study cohort was similar to that in a contemporary Australian population study (Ward et al, 2019), the authors are unable to suggest that the cohort is representative of the Australian obstetric population as a whole. The data were collected in the course of routine care, without the rigorous data verification that can be performed in prospective studies. The authors attempted to minimise this limitation during the analysis and verified the data by removing out-of-range data. Considering the retrospective nature of the study, data on gestational weight gain were not available, thus, the authors were unable to account for this variable in the regression analysis.
The major strength of the study is the use of a large dataset consistently collected over a period of 7 years in a large tertiary hospital with a very low number of women with missing maternal BMI data (3.6%). Although the study is not population-based, the hospital provides care to more than 5 500 pregnant women each year across a broad risk profile. Previous research has shown that classifying antenatal BMI based on self-reported weight and height is more likely to underestimate the BMI (Fattah et al, 2009). The BMI data used here were accurately measured by qualified clinicians (general practitioners and midwives) at the first antenatal visit, eliminating the risk of self-reporting errors.
Recommendations for practice
These findings have implications for maternity services and public health policies. Women need to be aware of the impact of overweight and obesity on themselves and their children. To optimise pregnancy outcomes, it is important to provide counselling at the initial prenatal and antenatal visits, and throughout the pregnancy, and discuss appropriate BMI and suitable diet and exercise. Overweight and obese women who intend to conceive should aim to lose weight before pregnancy and minimise weight gain during pregnancy to a level that does not negatively affect fetal growth and development (Tanentsapf et al, 2011). Management of antenatal weight in overweight and obese woman, as well as balancing the risks of fetal growth and obstetric complications, are based on clinical judgment of the healthcare provider, and need to be individually tailored to meet the needs of individual women.
Despite these recommendations, it has been reported that a large volume of interventions before, during and after pregnancy may have little impact on reducing the rate of maternal obesity or enhancing pregnancy outcomes for women with a high BMI (Tanentsapf et al, 2011; Hanson et al, 2017), unless other risk factors, such as maternal age, parity and medical conditions, are taken into consideration (Reynolds et al, 2019). This confirms the recommendations by the Australian Government (Commonwealth of Australia, 2019) emphasising the implementation of a broad range of multifaceted, community wide preventive health action plans to tackle overweight and obesity.
Conclusions
This study shows an upward trend in maternal high BMI in early pregnancy from 2011–2017 in a tertiary hospital with associated increases in assisted conception and caesarean section. Consideration of this trend and associations are important during the provision of pregnancy care, which is an ideal time to engage families and introduce health-improvement measures. Early identification of high BMI before or during the early weeks of pregnancy, and effective intervention, can help tailor health services to the needs of the population and provide sustainable, vigorous maternity care services, which in turn pave the way for healthy communities.
Key points
- Incidence of high body mass index (BMI) at early pregnancy increased by 3% annually from 2011−2017
- The risk of high BMI was greater in women who were older than 35 years of age, multiparous, were smoking, and with neurological disorders
- High early pregnancy BMI was associated with greater risks of having an assisted conception, a caesarean section and a larger neonate but smaller risks of episiotomy and neonatal admission to the special care nursery
- Community wide preventive health action plans are required to enhance women's awareness and provide counselling at initial prenatal visits and throughout pregnancy.


