Skin-to-skin contact (SSC), the deliberate placement of an infant on the bare chest of its mother, is commonly performed after normal vaginal delivery because of its numerous benefits (Moore et al, 2016). In contrast to the well-established evidence base after normal delivery, there is a paucity of high-quality clinical evidence for SSC after caesarean section (CS).
Despite the lack of evidence, the global prevalence of CS is elevated in developed countries, while the demand by mothers for SSC after CS has also increased as they strive to enhance their birth experience similar to a normal delivery (Phillips, 2013). The impetus to introduce SSC in mothers following CS is a consequence of a steadily increasing CS rate, especially in developed countries, with some areas of the USA and Ireland reporting a CS prevalence of 35% (Mangan et al, 2012). According to the NHS (2019), the CS rate in the UK for 2019 was 28%. The number of women performing SSC at birth following CS in UK and Ireland is not available. This number in USA facilities varies between 24–83% (Centers for Disease Control and Prevention, 2018), suggesting a cohort of women and infants are not receiving the potential benefits associated with performing SSC at birth during CS.
In a Japanese-Australian study, approximately 50% of the Japanese participants and 95% of the Australian participants had their SSC interrupted by hospital staff before completing one hour of uninterrupted SSC after CS (Brymdir et al, 2017). Similar interruptions can occur after vaginal birth too, as reported in the Cadwell et al (2018) cross-sectional study (n=84), in which only 63% of participants received immediate SSC as planned and only 27.4% started early breastfeeding.
Mangan et al (2012) reported several perceived barriers to performing perioperative in birth SSD, which include emergency CS, the inability to safely position the newborn on the mother's chest while they are lying flat on the operating table, the inability of the neonatal nurse to assess the wellbeing of the newborn while perioperative in birth SSC is taking place, and a low ambient temperature in the operating room.
Additional barriers reported in the Moore et al (2016) systematic review include the increased risk for newborn hypothermia secondary to cold operating room temperatures, as well as staffing issues and cost/time concerns, which can prevent theatre staff from promoting SSC after CS in theatre departments. Three clinical audits, which were conducted in the theatre department of a large Irish maternity hospital, also showed that over 80% (n=33/40) of mothers and 25% of neonates become hypothermic during or following CS, despite adherence to hospital guidelines for perioperative temperature control (Vilinsky and McCaul, 2017). The prevalence of perioperative maternal and neonatal hypothermia during SSC is an obstacle to theatre staff in maintaining and establishing undisturbed SSC and early breastfeeding within the theatre department. In many instances, mothers and newborns have to be separated to be warmed up, since hypothermia can increase morbidity in both mothers and their newborns (Bajwa, 2014).
Although growing in popularity, there are many obstacles that inhibit the initiation of SSC after CS (Smith et al, 2008; Hung and Berg, 2011). An important concern that is not yet adequately addressed by the literature and that SSC may directly influence is the potential for an increase or decrease in the incidence of neonatal hypothermia in the operating room (OR) if SSC is used. Although the evidence suggests that SSC promotes neonatal normothermia, this evidence is heavily reliant on studies after vaginal birth and not after CS (Vilinsky and Sheridan, 2014). Evidence from relatively small observational studies has identified that both mothers and newborns may become hypothermic during or after a CS in the absence of active preventative measures (Vilinsky and McCaul, 2017). Suboptimal maternal temperatures could potentially negatively impact neonatal outcomes through physiological heat loss mechanisms that occur in newborns (Ringer, 2013). Suboptimal neonatal temperature levels have adverse physiological effects in newborn infants, with the potential to progress to pathological changes, depending on the severity and duration of hypothermia (Bajwa, 2014). This is a narrative review of SSC following CS, which highlights the challenges encountered. One of those is hypothermia of mother and newborn, which impacts on the ability of mothers to undertake or continue SSC. This review also synopsises the adverse effects of hypothermia in neonates, explains the physiology of peripartum thermoregulation, and the mechanisms of heat loss and their prevention.
Search strategy
The search strategy used to identify the relevant literature for this review took place prior to this review being conducted using a Population, Exposure, Outcome (PEO) framework. The electronic bibliographic databases searched included EMBASE, PubMed, CINAHL, Cochrane and Google Scholar. Three separate and thorough searches were conducted. The first focused on SSC and its benefits, both after vaginal birth and CS, in term and preterm newborns. The second concentrated on the physiology of both adult and newborn thermogenesis, thermoregulation, heat loss mechanisms and hypothermia. The third was centred on active warming methods for preventing and managing perioperative maternal and neonatal hypothermia.
The search was limited to publications between 1985 and 2019 that were published in the English language, as studies reviewing the physiology of thermoregulation on fetuses and newborns were initially conducted in the mid-1980s. MeSH and non-MeSH key words were used in the above searches (Box 1). The above search terms were combined where appropriate using the AND/OR Boolean operators. After the initial search, 468 articles were entered in EndNote. The software identified 146 duplicates. After the removal of the duplicates, all articles were reviewed individually. A total of 214 articles were found to be irrelevant to the topic and were removed, leaving 108 relevant articles. A total of 22 were discarded at this stage, as they were older publications and it was the authors' decision to keep only the latest knowledge from the field. A total of 86 articles were included in this review.
Box 1.Key words used in literature search
- Skin-to-skin contact (SSC)
- Kangaroo Care
- Neonatal bonding
- Benefits of SSC
- Obstacles/barriers for SSC
- Fetal/neonatal/maternal thermoregulation
- Thermogenesis
- Neonatal hypothermia
- Maternal hypothermia
- Inadvertent perioperative hypothermia
- Prevention of hypothermia
- Heat loss mechanisms
- Operative birth
- Cesarean section (CS)
- Caesarean section
- Operative delivery
- CS rates in Europe
- CS global rates
- Perioperative active warming
- Warm IV fluids
- Forced-air warming device
Skin-to-skin contact
Benefits
SSC is defined as the practice of positioning the newborn, dressed only with a nappy and a hat, on its mother's bare chest, covered only with warm dry towels/blankets (World Health Organization [WHO] and United Nations Children's Fund, 2009). SSC after vaginal deliveries has been researched over the past 25 years, and it is growing in popularity because of the significant benefits it has for both the newborn and mother (Moore et al, 2016). SSC can be described as ‘at birth’ or immediate SSC (when it is initiated within 10 minutes after birth) and early SSC (initiated between 10 minutes and 24 hours after birth) (Moore et al, 2016).
A number of the randomised controlled trials (RCTs) included within the review investigated the benefits of at birth SSC in both mothers and newborns. All revealed positive outcomes for the immediate initiation of SSC. Armbrust et al (2016) conducted a study involving 205 mothers, and found that parental birth experiences and breastfeeding rates after a CS were significantly higher in the group that performed SSC (P<0.05). Schneider et al (2017) (n=2841) found that newborns who performed SSC following a CS birth had significantly lower risk of being admitted to the Neonatal Intensive Care Unit (NICU), compared to newborns who did not perform SSC (P=0.001). Huang et al (2019) (n=108) reported that newborns delivered by CS who performed at birth SSC had a more stable heart rate, higher forehead temperature, less duration of crying, earlier initiation of feeding behaviour, and longer duration of breastfeeding, while fathers who performed SSC had lower scores of anxiety, depression and better role attainment than those who did not perform any SSC. Benefits associated with SSC are outlined in Table 1. Kollman et al (2017) (n=35) studied at birth SSC compared to late SSC, following CS. This study revealed no significant differences between the groups on the measured outcomes, which included neonatal stress response (P=0.762), maternal salivary cortisol (P=0.471) and pain (P=0.317).
Table 1. Benefits of skin-to-skin contact
| Lowers the risk of neonatal hypothermia |
| Increases mean neonatal body temperature |
| Reduces the risk of neonatal infection |
| Reduces the risk of neonatal sepsis |
| Prolongs the duration of breastfeeding |
| Reduces the risk of hypoglycaemia |
| Reduces tachypnea |
| Improves oxyhaemoglobin saturation |
| Reduces maternal anxiety reduces early depression |
A meta-analysis (Boundy et al, 2016) on the effects of SCC on neonatal wellbeing, which included 124 studies, found that one of the main benefits of SSC was a decreased risk of neonatal hypothermia (relative risk [RR]=0.22; 95% confidence interval [CI]=0.12–0.41, P>0.01) and hypoglycaemia (RR=0.12; 95% CI=0.05–0.32, P>0.01). The mean body temperature of newborns who had SSC was 0.24°C higher than the control group (n=14; 95% CI=0.15–0.33; I2=82%). This is consistent with the findings of a systematic review by Moore et al (2016), which included 46 studies (8/46 addressed SSC following elective CS in term neonates). This review found that SSC increased the exclusive breastfeeding rates at hospital discharge and at 1–4 months post-delivery (RR=1.24; 95% CI=1.07–1.43) and that newborns who performed SSC had higher blood sugar levels 1.5 hours after birth (mean difference (MD)=10.49 mg/dL; 95% CI=8.39–12.59). Furthermore, breast engorgement pain on day three post-delivery was lower in mothers who performed SSC (standard mean diffference (SMD)=-0.41; 95% CI=-0.76–-0.06). Finally, SSC reduces feelings of anxiety (SMD=-0.32; 95% CI=-0.59–-0.04) for mothers on day three post-delivery.
Although SSC has a number of benefits for both mothers and newborns (including reducing the incidence of neonatal hypothermia), most studies focused on women following vaginal births. There are a limited number of studies reviewing SSC during or after elective CS, out of which only five focused on neonatal and maternal temperatures (Huang et al, 2006; Gouchon et al, 2010; Horn et al, 2014; Paris et al, 2014; Vilinsky et al, 2016). One of the main reasons for the limited research in this particular cohort of mothers is that SSC during CS is a new concept, with recent attempts being made to implement it in theatre (Phillips, 2013). The small number of papers found on perioperative neonatal and maternal hypothermia during CS while at birth SSC is performed indicate the lack of research on this topic and guided the current review to focus on core issues that emerge from the literature.
Risks
A number of case studies published to date report potential risks, including neonatal Sudden Unexpected Postnatal Collapse (SUPC), falls, and suffocation of newborns performing SSC (Feldman et al, 2016). The common denominators in these case studies were the prone position of newborns during SSC and primigravida mothers with high body mass index (BMI) who were unsupervised by hospital staff during the SSC period (Matthijsse et al, 2016; Feldman-Winter et al, 2016). However, a number of other studies suggested that newborns undertaking SSC are less at risk of falls and suffocation than infants placed in a prone position elsewhere (Singh et al, 2012; Ludington-Hoe and Morgan, 2014). Nevertheless, regular neonatal observation by the hospital staff, especially during the first 2 hours post-delivery, is crucial in reducing the risk of neonatal fall and suffocation, regardless of whether SSC is performed or not (Dageville et al, 2008; Ludington-Hoe and Morgan, 2014).
Neonatal hypothermia
Neonatal hypothermia is defined as a pathological condition in which the newborn's body temperature drops below 36.5 °C (Kumar et al, 2009), while normothermia is the normal range of core temperature between 36.5–37.5 °C. Categorising of neonatal hypothermia remains unchanged from when the WHO (1997) categorised neonatal hypothermia into three core temperature groups:
- Mild hypothermia (or cold stress): 36.0–36.4 °C
- Moderate hypothermia: 32.0–35.9 °C
- Severe hypothermia: <32.0 °C.
Mechanisms
Newborn thermoregulation is heavily dependent on environmental temperature, the health status of the newborn and a number of heat production and heat loss mechanisms (Ringer, 2013). According to WHO (1997), the environmental temperature of the delivery room should be kept at a minimum of 25°C for a term baby with normal birth weight and 26-28°C for preterm babies or low birth weight babies. Neonatal heat production mechanisms (thermogenesis, Table 2) include metabolic processes, voluntarily muscle activity, peripheral vasoconstriction and non-shivering thermogenesis (Leduc and Woods, 2017). Newborns are prone to rapid heat loss, resulting in hypothermia, as a result of their high surface area to volume ratio, thin skin with blood vessels close to the surface and reduced subcutaneous fat (Leifer, 2013; Gibson and Nawab, 2015). The core temperature can decrease by 0.1°C per minute while skin temperature can decrease by 0.3°C per minute if no actions are taken (Waldron and Mackinnon, 2007). There are four main heat loss mechanisms in the human body: conduction, evaporation, radiation and convection, which affect adults and newborns alike (Leifer, 2013; Ringer, 2013).
Table 2. Neonatal heat production mechanisms
| Metabolic processes | Main organs, such as the brain, the heart and the liver, produce energy by metabolising glucose, fat and protein |
| Voluntary muscle activity | Generated during restlessness and crying, which generates heat through increased muscle activity |
| Peripheral vasoconstriction | Refers to constriction of the blood vessels close to the skin and the extremities, as a response to cooling, reducing the blood flow to the skin and decreasing loss of heat from the skin's surface |
| Non-shivering thermogenesis | Brown Adipose Tissue (BAT) is metabolised to generate heat. BAT is a fatty tissue located around the kidneys, head, neck, heart, great vessels/adrenal glands and axillary regions |
Pathophysiology
Neonatal hypothermia may affect a number of different systems in the newborn's body, including the cardiopulmonary, central nervous and vascular systems (Mank et al, 2016) (Figure 1). Adverse cardiovascular effects include a fall in systemic arterial pressure, decreased plasma volume, decreased cardiac output, and increased peripheral resistance (Mank et al, 2016). Severe hypothermia can lead to bradycardia and tachypnoea, which, if left unchecked, can lead to permanent tissue damage, neurological impairment, or death (Bajwa, 2014). Lesser degrees of hypothermia (cold stress) can lead to vasoconstriction; in more severe cases, this can reduce blood flow, and therefore tissue perfusion to skin and other organs, leading to hypoxia and eventually acidosis (decrease of blood pH) (Leifer, 2013). Physiological compensation occurs via tachypnea, which increases oxygen demand. Energy demand increases in parallel, which leads to increased anaerobic metabolism and metabolic acidosis once glycogen stores are depleted. (Leifer, 2013). Hypothermia may also reduce surfactant production, causing alveolar collapse and adversely impacting lung function with subsequent hypoxia (Thornton et al, 2015). In full term infants, hypoxaemia interferes with physiologic ductus arteriosus closure (foetal circulation). Reduced renal perfusion may result in renal impairment and fluid retention, which may contribute to congestive heart failure (Volpe et al, 2017). Likewise, reduced blood flow to the intestines (intestinal ischaemia) may lead to feeding intolerance and necrotizing enterocolitis (Gournay, 2011). Finally, the consumption of brown adipose tissue (BAT), to generate heat and raise the body temperature, releases fatty acids into the newborn's bloodstream, potentially interfering with the transport of bilirubin to the liver, resulting in hyperbilirubinemia and jaundice (Leifer, 2013). Based on current literature, neonatal hypothermia has a significant impact on multiple newborn systems, including cardiovascular, pulmonary, gastrointestinal and renal. Such an impact has the potential to lead to disjunction of the previously mentioned systems, and therefore interfere with the wellbeing of an otherwise healthy newborn. This suggests that neonatal hypothermia should be prevented after birth. Neonatal hypothermia can be prevented using either passive or active warming (Holtzclaw, 2008). Passive warming includes newborn caps and plastic bag wraps, while active warming refers to radiant heaters, exothermic mattresses and SSC (Holtzclaw, 2008; McCall et al, 2018). According to current literature, SSC could be used as an effective, affordable and readily available method used to promote normothermia and prevent hypothermia during or after vaginal delivery.
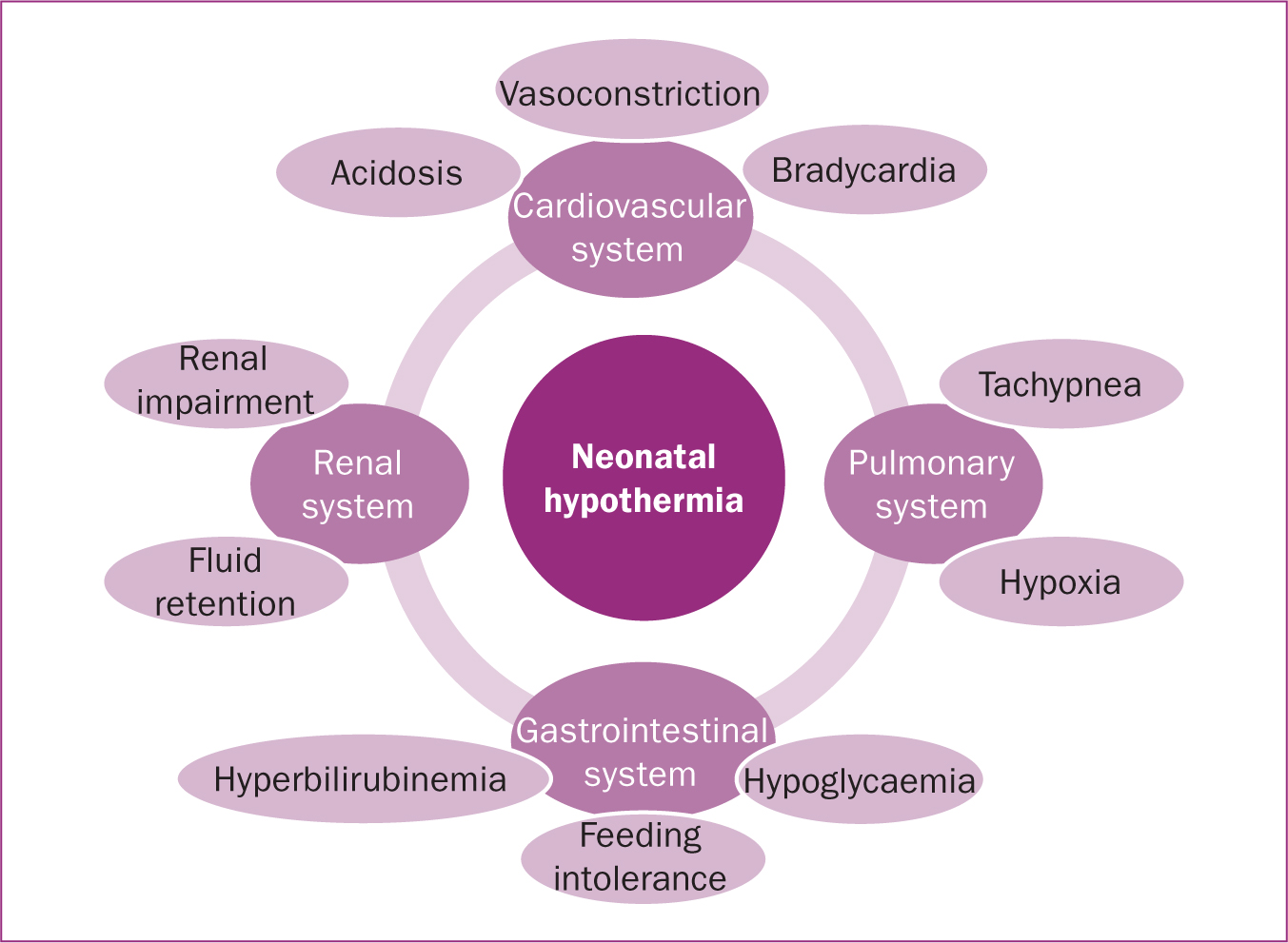 Figure 1. Pathophysiology of neonatal hypothermia
Figure 1. Pathophysiology of neonatal hypothermia
Maternal hypothermia
Hypothermia in pregnant women is defined as a core temperature below 36°C (Desgranges et al, 2017). Mild hypothermia (temperature ranging between 35.9°C and 35°C) is a frequent occurrence among pregnant women undergoing CS (Munday et al, 2014; Desgranges et al, 2017). Inadvertent perioperative hypothermia (IPH) may increase the morbidities experienced by mothers after CS (Sultan et al, 2015). It is found that more than 60% of women who have a CS will develop hypothermia (Cobb et al, 2016). Hypothermia has been associated with shivering, delayed wound healing and increased risk of infection (Melling et al, 2001; Cobb et al, 2018). When severe, it decreases platelet activity and increases bleeding risk (Rajagopalan et al, 2008). Additional effects of IPH include longer post-anaesthetic recovery, and longer stay in hospital (Karalapillai et al, 2013), altered drug metabolism (McSwain et al, 2015) and patient thermal discomfort (Alfonsi et al, 2003). Unfortunately, the effect of maternal hypothermia on neonatal temperature is another area not adequately researched, especially when SSC during CS is performed.
Prevention of maternal inadvertent perioperative hypothermia
The use of preoperative warming alone is not adequate to prevent maternal IPH during and after CS. Therefore, a combination of warm IV fluids administration and forced air warming (both of which are active warming methods) should be considered (Munday et al, 2018). Intraoperative warming of the pregnant women undergoing CS could take place with the use of active warming devices, such as fluid warmers and forced air warming (National Collaborating Centre for Nursing and Supportive Care, 2008).
A fluid warmer involves actively warming intravenous (IV) fluids with the use of various fluid warming devices prior to fluid IV administration to the patient. In forced air warming, a specially designed blanket is applied over the pregnant woman's half body (upper or lower) and warm air is forced through the device to the equipped blanket for as long as the device is activated. A meta-analysis by Sultan et al (2015) involving 13 RCT studies (n=789 pregnant women undergoing elective CS) compared the use of perioperative active warming versus no warming. The findings suggested that the use of warm IV fluids and forced air-warming increased maternal temperature at the end of surgery (MD=0.43°C; Cl 95%=0.27–0.59; P<0.00001), reduced shivering (RR=0.58; Cl 95%=0.43–0.79; P=0.0004), increased thermal comfort (SMD=0.90; Cl 95%=0.36–1.45; P=0.001) and decreased maternal hypothermia (RR=0.66; Cl 95%=0.50–0.87; P=0.003). Notably, these studies inconsistently reported neonatal temperatures and did not report on SSC-related outcomes. There is also a lack of evidence on the effect of perioperative maternal active warming during elective CS on neonatal hypothermia when SSC in birth is performed, suggesting the need for conducting further research in this field.
Guidelines for preventing inadvertent perioperative hypothermia
The only published guidelines on prevention of IPH are available from NICE (2016); however, they are focused on the general adult surgery population and exclude pregnant women and children/newborns. Following a database search (PubMed, CINAHL, Embase), including a Google search for additional national or international guidelines, no extra or relevant guidelines pertaining to pregnant women developed by other organisations were found. This dearth of evidence suggests that each hospital has its own method of dealing with this issue, based on individual hospitals policy rather than evidence-based practice.
Conclusions
This narrative literature review suggests that although SSC is a beneficial practice and promotes neonatal normothermia after vaginal birth, it is still not well established during CS, because of a number of obstacles, including increased prevalence of neonatal/maternal hypothermia, within the theatre departments. The review suggests that women and newborns are prone to becoming hypothermic during CS if active preventative measures are not implemented. This contributes to potentially avoidable maternal and neonatal morbidity. Available research is insufficient to conclusively determine a causal link between perioperative maternal temperature management and neonatal hypothermia during/after SSC at birth in women undergoing CS. The optimal strategy for active warming is not known. Additionally, current international guidelines focus on the general surgical population, while pregnant women and newborns have been excluded from these guidelines because of a lack of evidence on the safety and efficiency of perioperative active warming in this specific population. This lack of evidence indicates the need for clinical research on this topic. An up-to-date systematic review and meta-analysis of RCTs comparing the effect of various maternal perioperative active warming methods versus no warming on the incidence of neonatal hypothermia during at birth SSC is warranted to address this gap in the evidence.
Implications
This literature synthesis confirms the benefits of SSC for both mothers and babies, and suggests a trend in promoting SSC during CS, particularly as the global rates of CS are increasing and more women show interest in having SSC during their operation. It also demonstrates that risk of hypothermia is a barrier to SSC during CS, as hypothermia, particularly when severe, is known to cause a number of morbidities in both mothers and their newborns during and/or after CS. Importantly, perioperative hypothermia is common and preventable. Available evidence suggests that for SSC to be safely promoted during CS, maternal and neonatal hypothermia should be prevented, preferably by a method that minimizes the incidence of maternal/newborn separation. Prevention of maternal hypothermia could be reduced using perioperative active warming methods, which maintain maternal temperature and placing a newborn for SSC on a warm mother. These combined steps could potentially reduce the risk of neonatal heat loss via conduction and therefore reduce the incidence of perioperative neonatal hypothermia during SSC. There is a need for a systematic review of existing RCTs on the effect of perioperative maternal active warming on neonatal hypothermia while at birth SSC is performed.
Key points
- There is an increased demand by mothers to perform skin-to-skin contact after caesarean section.
- There is an increased risk of maternal and neonatal hypothermia during and after caesarean section.
- Although evidence suggest that skin-to-skin contact promotes neonatal normothermia, it is based on studies performed during vaginal birth.
- This narrative review focuses on the available evidence for skin-to-skin contact after caesarean section, the adverse effects of hypothermia, and the mechanisms of heat loss and its prevention during caesarean section


