Gastro-oesophageal reflux (GOR) is a commonly reported phenomenon encountered in the initial weeks of neonatal life, and is a normal physiological process which usually occurs following feeding (National Institute for Health and Care Excellence (NICE), 2015). However, GOR can cause distress and concern at an already challenging time during the transition to parenthood, often necessitating additional multidisciplinary support. It is a common condition, which only becomes problematic if symptoms are extreme enough to warrant treatment. Midwives may be the first practitioners with whom parents discuss feeding issues, and therefore are well-placed to counsel parents about the normality of GOR and when the condition becomes pathological GORD (gastro-oesophageal reflux disease) requiring intervention or treatment. Therefore, it is imperative that midwives can sensitively and knowledgeably interpret parents' communications about their babies' feeding habits to offer either minimal midwifery-based or multidisciplinary solutions once additional support is indicated.
Definition
To be able to provide appropriate reassurances to parents, it is important that all health professionals use clearly defined terminology. However, historically there has been great variation surrounding the definitions of GOR and GORD, with terms often being used interchangeably (NICE, 2015) (Figure 1). With disparity between professional classifications evident, it therefore becomes difficult to effectively communicate to parents, making the need for clarity all the more important.
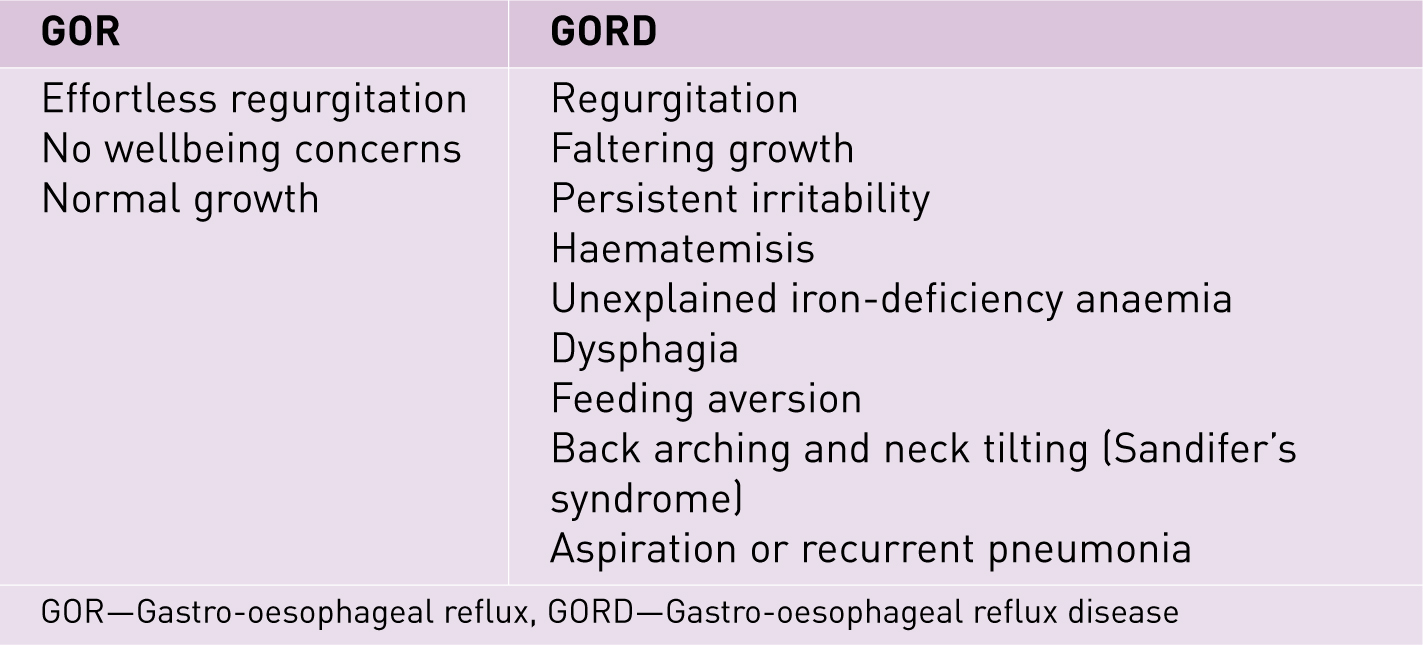
GOR
GOR is defined as the normal physiological process whereby gastric contents pass into the oesophagus effortlessly, which may, or may not, be accompanied by retrograde movement, regurgitation and vomiting (Hyman et al, 2006; Vandenplas et al, 2009). GOR has no presenting complications where the neonate is generally well (Dogra et al, 2011), and can be differentiated from vomiting. Vomiting is a central nervous system response involving the use of autonomic and skeletal muscles whereby gastric contents are forcefully expelled due to coordinated movements from the small bowel, stomach, oesophagus and diaphragm (Hyman et al, 2006), whereas GOR is an effortless motion.
GORD
GORD occurs when this normal reflux of gastric contents causes complications or symptoms, which may be upsetting and worrying for parents and uncomfortable and distressing for the neonate (Vandenplas et al, 2009). Recently published NICE guidance for GOR and GORD suggests that medical investigation or intervention should only be necessitated if discomfort levels with associated complications arise, unexplained feeding difficulties occur, growth falters, or neonatal wellbeing otherwise deteriorates (NICE, 2015).
Babies will display irritability, failure to thrive, insufficient calorific intake, sleep disturbances including apnoea, respiratory symptoms, haematemesis, swift termination of feeds and weight loss in moderate-to-severe cases of GORD (Van Wijk et al, 2007; Poets and Brockmann, 2011; Cremonesini, 2014) with reports of ‘excessive’ GOR estimated at around 8% of healthy infants (Huang et al, 2009). Because there is no definitive test, diagnosis becomes dependent on practitioner assessment and clinical presentation, which can vary widely, making incidence and treatment management problematic, posing challenges for many families and practitioners, thus causing large economic repercussions for health services (NICE, 2015).
Incidence
Around 50% of babies up to 3 months old regurgitate at least once a day (Nelson et al, 1997; Tighe et al, 2014), and are often referred to as the ‘happy spitters’ (Corvaglia et al, 2013). GOR can be considered a normal physiological part of the digestive process, often happening post-feed (NICE, 2015). This usually improves with age, with symptoms disappearing in 55% of infants aged 10 months, 81% of infants aged 18 months, and 98% of infants aged 2 years (Vandenplas et al, 2009). Similarly, recurrent regurgitation also improves with age, with a peak incidence of 67% at 4 months (Dogra et al, 2011) followed by a steady decline in incidence to around 5% in 10–12 month-old infants (Vandenplas et al, 2009). Most cases of GOR can therefore be considered self-limiting and seem to be rectified by 1 year without complication as part of normal neonatal development (Tighe et al, 2014).
Although otherwise well infants can experience GORD, the risk of GORD is increased in premature neonates, low birthweight babies and infants who have complex and severe neurological impairment or neurodisabilities (Vandenplas et al, 2009). This is due to a significantly immature anti-reflux mechanism (Richards et al, 2014). Other childhood diagnoses associated with an increased risk of GORD include oesophageal atresia with repair, hiatus hernia, bronchopulmonary dysplasia, cystic fibrosis and asthma (Jung, 2001).
Anatomy and physiology
GOR is thought to occur due to several physiological factors. The major contributor is thought to be the transient lower oesophageal sphincter relaxation (TLOSR) due to immature muscular development, leading to stomach contents being regurgitated and passing into the oesophagus (Omari et al, 2002; Corvaglia et al, 2013). This represents the main pathogenic mechanism in preterm infants and is linked to 92–4% of GOR episodes in these babies (Omari et al, 2002). Omari et al (2002) found that infants experiencing GOR had a higher proportion of TLOSR associated with acid reflux. However, no difference was observed in the frequency of TLOSR episodes between healthy infants and those affected by GOR, an unexpected finding which suggests that other physiological mechanisms may also be involved in the underpinning physiology (Omari et al, 2002). Components also believed to be involved in GOR include laying babies in the supine position following feeds, which is thought to promote the rise of gastric contents into the oesophagus, relatively abundant milk intakes, and the immaturity of oesophageal motility, which entails a poor clearance of refluxate especially in the preterm population (Corvaglia et al, 2013).
Symptom recognition and midwifery management
Midwifery care during the puerperium offers a unique opportunity to holistically assess neonatal feeding patterns and mother–baby wellbeing. From the initial postnatal visit, midwives continually assess neonatal wellbeing by recognising symptoms deviating from the normal and reassuring parents regarding the normal feeding behaviour of their infant (NICE, 2014). GOR usually presents prior to 8 weeks of age, with 5% of babies experiencing six or more episodes of reflux daily (NICE, 2015). Midwives are often the first professional accessed for information and advice, and thus discussion should be based on a thorough history including gestation at birth, method and mode of feeding (i.e. breastfeeding or artificially feeding via a cup, bottle, or nasogastric tube) and a rigorous assessment of neonatal wellbeing should be undertaken.
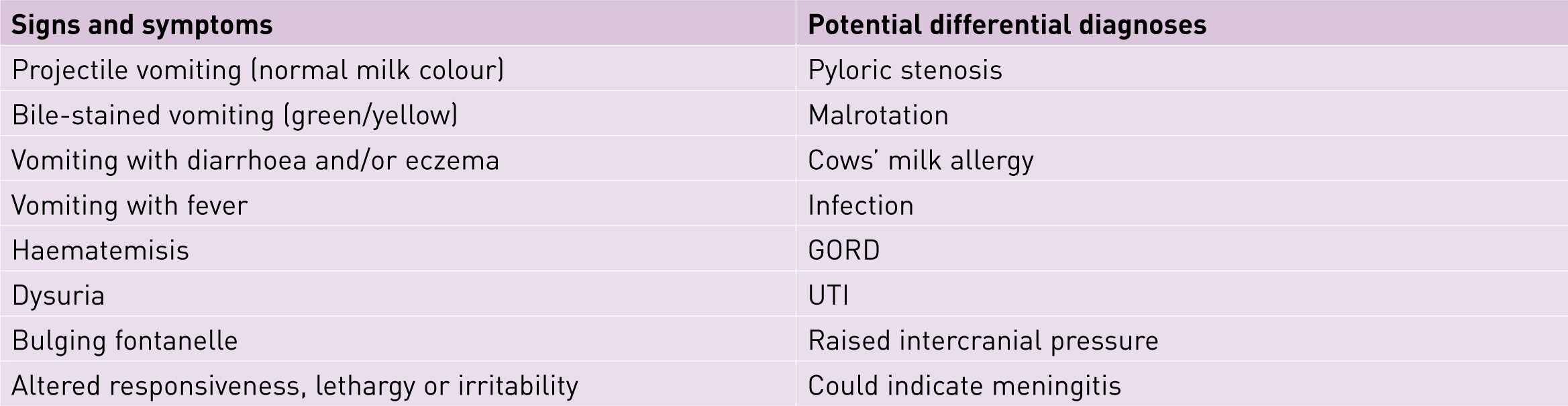
Current literature emphasises the need to offer advice situating GOR as a normal part of neonatal physiology and it is important to communicate that unlike GORD, it is not necessarily detrimental to neonates (Vandenplas et al, 2009). Effective reassurance includes an empathetic and evidence-based response to concerns, and an assurance of continuing availability of health professionals, with parents encouraged to seek reassessment and access support at any point (Hyman et al, 2006; Raynor and England, 2010). GOR may be shocking for new parents and requires sensitive enquiry as to the nature of parental concern. Because GORD has such a wide-ranging symptom base, all-inclusive history-taking of feeds is essential because this formulates the basis for any referral pathway or intervention (Scholler and Nittur, 2012). Midwives must be cautious about differential diagnoses (Figure 2), considering the intricate clinical picture and liaising with the multidisciplinary team should any concerns be noted. This is particularly relevant when mothers and babies move from midwifery service provision into accessing care in a community setting via GPs and health visitors, with feeding issues including GOR related to poor weight-gain being reported in some studies among the highest reasons for neonatal readmissions (Roth et al, 2010). Parents may present to the general practitioner or health visitor with their concerns, and therefore effective team working across disciplines and interdepartmental communication is essential to ensure that parents do not receive conflicting advice.
GOR can be difficult for parents to deal with, especially during the early stages of parenthood. Additional risk factors, such as having experienced a traumatic pregnancy or birth or having a history of anxiety or depression, may exacerbate these challenges. Parents may report that their baby is irritable and they may feel helpless and unable to console the baby (Radesky et al, 2013; Cremonesini, 2014). If this irritability continues it can create depressive symptoms and negatively impact mother–child bonding and attachment (Cremonesini, 2014), which can have a long-term effect on babies and parents and potentially lead to an unwanted change of feeding method (Ystrom, 2012). The importance of midwives and other health professionals assuaging anxiety and alleviating parental guilt is paramount to enable the parents to continue to foster a positive relationship with their baby.
‘Feeding is often highly emotive and complex for families, and accommodating maternal choice while ensuring neonatal wellbeing can be challenging for practitioners.’
Feeding
Considering the holistic feeding picture is essential to be able to accurately advise parents and ensure an individualised care plan is implemented to improve GOR. Feeding is often highly emotive and complex for families, and accommodating maternal choice while ensuring neonatal wellbeing can be challenging for practitioners.
For infants who are being fed with expressed milk or formula milk, individual requirements should be calculated to assess whether babies are feeding excessive amounts for their weight. If they are feeding excessively, simply reducing feed volumes could have a marked effect on GOR (NICE, 2015). If infants are taking an appropriate amount of feed, then trialling smaller, but more frequent, feeds while maintaining the same overall intake may reduce symptoms. There is currently no robust evidence to show benefits for using feed thickeners to aid treatment in infants (Huang et al, 2009). However, if altering feeding patterns has not alleviated symptoms or improved neonatal wellbeing, it may be worth considering implementing this (NICE, 2015).
‘There is no singular, consistent, accurate diagnostic test that can differentiate between GOR and GORD’
Any neonatal or maternal wellbeing concerns when the woman is breastfeeding should be followed up by a practitioner who is confident in providing breastfeeding support carrying out a breastfeeding assessment (Entwistle, 2013). This includes observing a full feed and paying particular attention to positioning, attachment and signs of effective milk transfer. Any issues with feeding may be corrected with the support of professionals, and signposting the woman to support groups, such as the Breastfeeding Network and La Leche League International can be helpful. It can be challenging to assess speed of milk intake at each breastfeed, and it is acknowledged that practitioners have little knowledge of how to support breastfeeding when the neonate is experiencing GOR (Vandenplas et al, 2009; Banna and Jutel, 2013). Advising parents to try smaller and more frequent feeds may still be beneficial, and weaning should not be recommended (Banna and Jutel, 2013). The benefits of breastfeeding are extensive both for mother and baby, and breastfeeding remains the optimum nutrition for infants, with the World Health Organization recommending exclusive breastfeeding for a minimum of 6 months (WHO, 2011).
The feeding picture can be especially multifaceted in the premature infant, especially those who are receiving additional nutritional input with complex feeding plans and perhaps more invasive modes of feeding, such as via a nasogastric tube. The midwife who is visiting these babies at home should refer any concerns immediately to the paediatric and infant feeding teams (Nursing and Midwifery Council (NMC), 2012). If the baby is significantly unwell or faltering in growth, alongside individualised feeding support, the baby may require initial supplementation of expressed milk, although donor milk and artificial milk supplements can also be explored. Considering the overall clinical picture is essential at this point, and gaining fully informed consent for any intervention is vital to determine long-term feeding outcomes. Enteral tube feeding should only be considered if babies have significant faltering growth which has no other explanation after other treatment strategies have been attempted (NICE, 2015).
Cows' milk allergy
Cows' milk allergy can be hard to distinguish from GOR as presentation may be similar, with symptoms often overlapping. However, cows' milk allergy can be confirmed after elimination of cows' milk protein from the diet results in decreased frequency of vomiting, with reintroduction causing a recurrence of symptoms (Vandenplas et al, 2009). If cows' milk allergy is suspected, it is recommended that there should be complete elimination of cows' milk from the diet (or from maternal diet if breastfeeding) for 2–3 weeks, with close observation as to whether symptoms resolve (Allen et al, 2009). This will usually confirm suspected cases, although it is important that parents are supported to disclose their concerns with a practitioner able to advise them accordingly, as some parents may self-diagnose a cows' milk allergy based on anecdotal advice from friends or family. If cows' milk is entirely eliminated from the diet with no attempt to reintroduce it, a diagnosis cannot then be confirmed.
Investigations
The diagnoses of GOR and GORD are made based on clinical signs and symptoms (Vandenplas et al, 2009), although different investigations may be considered to assist the practitioner in making the diagnosis. However, there is no singular, consistent, accurate diagnostic test that can differentiate between GOR and GORD, which impacts on clinical diagnosis and decision-making (NICE, 2015).
Twenty-four hour oesophageal pH-monitoring can quantify reflux, evaluate atypical symptoms and monitor medical treatment (Vandenplas et al, 2009). The process of carrying out this investigation includes inserting a probe into the oesophagus to measure both frequency and duration of acid exposure (Jung, 2001). Barium contrast studies are readily available and can be used to identify anatomical abnormalities in the upper gastrointestinal structure, although they are inadequate to diagnose GORD (Jung, 2001) and are not recommended in infants (NICE, 2015). If GORD is persistent, endoscopy with a biopsy can be used to evaluate GORD, although as an invasive procedure it requires sedation (Jung, 2001; Vandenplas et al, 2009) and therefore should only be considered if there are significant concerns around neonatal wellbeing.
Management strategies
The aims of any management strategy of GORD are to alleviate symptoms, promote normal growth and weight-gain and prevent complications (Mir, 2010).
Conservative management is considered to be the best option for GOR, and it can alleviate symptoms of GORD. This includes reassuring the parents that the natural progression of GOR in most cases is to improve with age, with 98% of infants experiencing symptom disappearance by 2 years of life (Vandenplas et al, 2009), and reassuring parents that GOR does not usually need any further investigation or treatment. However, parents should be made aware of symptoms of deteriorating neonatal wellbeing, such as projectile regurgitation, bile-stained vomiting or haematemesis, or any signs of marked distress or faltering growth, and should be encouraged to seek additional support should these occur (NICE, 2015).
Neonatal positioning following feeding is often promoted as a conservative management option. Van Wijk et al's (2007) findings suggested that a left lateral position over right lateral body position decreased the trigger of liquid reflux episodes after feeding. A more prone post-feed position is also thought to lessen the instance of GOR; however, this has repercussions in terms of infant sleep and risk of sudden infant death syndrome. Infants should always be placed supine for sleeping in accordance with Department of Health guidance (DH, 2009) and so any discussion with parents must stress the importance of a supine position for sleeping (Ferreira et al, 2014).
The evidence underpinning pharmacological intervention for GORD is poor (Tighe et al, 2014), with use of varying pharmacological managements often depending on practitioner preference and experience.
Alginate therapies, such as Gaviscon Infant® are commonly used to treat the symptoms of GOR when changes in feeding are unsuccessful, following the cessation of thickening agents (Tighe et al, 2014). Alginate therapy is easily accessible and parents often identify this as a first-line treatment (Cremonesini, 2014). Alginates work by mixing with milk in the stomach to create a more viscous substance, thus preventing reflux, although anecdotally effects are limited (Cremonesini, 2014). Alginates should initially be offered for trial periods of 1–2 weeks. If therapy is beneficial, then this can continue, although should be stopped at regular intervals to assess whether the infant has recovered from GOR (NICE, 2015).
Proton pump inhibitors (PPI), such as omeprazole, work by reducing the amount of acid in the stomach, although these should be avoided if regurgitation occurs as an isolated symptom (NICE, 2015). Antacids, such as ranitidine, are also frequently used to treat the symptoms of GOR, although similarly, treatment should be withdrawn at regular intervals to assess whether the infant still requires intervention (NICE, 2015). Antacids are considered a safe form of treatment for GOR as their side effects are significantly less than pharmacological treatments such as domperidone (Bhasvar et al, 2011; Cremonesini, 2014).
Domperidone is a prokinetic agent, which increases the oesophageal sphincter pressure and increases the motility of the gut, although there is little research to assess its efficacy (Cremonesini, 2014). However, it is associated with a variety of unpleasant and damaging side effects, including an increased QT prolongation and cardiac arrhythmia which could be potentially fatal (Perrio et al, 2007; Viera et al, 2012). Therefore, the side effects of domperidone are widely advised to outweigh its potential for benefit (Vandenplas et al, 2009).
Surgical management of GORD is reserved for only the most severe of cases and involves fundoplication, an operation whereby the fundus is wrapped around the base of the oesophagus and stitched in order to tighten the oesophageal sphincter (Hassall, 2005; Cremonesini, 2014).
Conclusion
Recently published NICE guidance (2015) provides a new framework improving evidence-based management for GOR and GORD, which can be applied across all disciplines. This creates the possibility for a joined-up approach using the most up-to-date evidence-base and could assist in assuaging confusion around early parental feeding concerns, as well providing clarity for management choices and referral pathways. Midwifery neonatal assessment is a fundamental skill throughout postnatal practice, and midwives must be able to proficiently assess wellbeing including feeding and subsequent arising issues which have an impact on early parenting days. Requiring considerable additional support from a wider multidisciplinary team, midwives can use their holistic family approach acknowledging that a failure to recognise and take swift action with GORD could lead to significant deterioration in neonatal wellbeing, maternal distress and a lack of maternal confidence in their ability to effectively feed their babies. In order to make individualised neonatal care plans in collaboration with parents, midwives, GPs and associated frontline health professionals must ensure they are equipped to understand and effectively communicate differences in symptom presentation and care pathways between GOR and GORD.

